Abstract
Summary: We describe a relatively unusual case of carotid cavernous fistula in association with a persistent trigeminal artery, presumably related to aneurysm rupture near the carotid origin of the vessel. We emphasize the use of a second, nondetachable balloon solely for the purpose of stabilizing placement of the first device at the time of detachment.
The concept of a catheter-delivered detachable balloon as a therapeutic cerebrovascular device is credited to Serbinenko (1). In North America, the technique (in various permutations) has been popularized by Debrun et al (2) and Heishima et al (3) for the treatment of direct carotid cavernous fistulas (CCFs) and by others for hunterian occlusion (after successful test occlusion) of giant, intracranial aneurysms (4–6). Although generally accepted as the method of choice for the treatment of direct CCFs, detachable balloon embolization in the CNS is a procedure of some complexity, with a potential for significant complications (7).
Many factors influencing outcome bear on the critical timing of balloon detachment. Previous reports have emphasized detachment of these devices by means of a second, coaxial (4F over 2F) sleeve, which pushes the balloon off the delivery catheter, or by means of simple inflation and gentle traction on the delivery catheter (3). Poor tracking of the sleeve and inability to push the balloon into place may hamper the former method. The second technique is fraught with the risk of balloon migration (particularly into the carotid artery) at the time of detachment. This report offers another method with which to control and stabilize balloon placement and detachment.
Technique
A 43-year-old right-handed woman, in previously good health, presented with a 2-month history of rapidly progressive pulsatile headache and bruit, proptosis, visual disturbance, and redness in the right eye. She had no history of trauma and no family history of connective tissue disorder or berry aneurysm. An MR imaging study performed at an outside institution revealed an enlarged area of flow void at the right cavernous sinus. The patient was then referred for angiography and possible embolization of a suspected CCF.
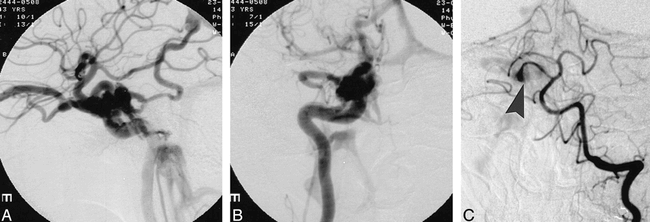
Cerebral angiography revealed a rapidly filling, direct CCF involving the C5 segment of the right carotid siphon (Fig 1A and B). Somewhat unexpectedly, filling was also seen with vertebral injections by way of a small persistent trigeminal artery on the right (Fig 1C).
A and B, Lateral (A) and anteroposterior (B) catheter angiograms show a direct CCF with rapid filling of the cavernous sinus, superior ophthalmic vein, and inferior petrosal sinus.
C, Anteroposterior view of the left vertebral artery injection similarly shows filling of the right cavernous sinus via a persistent trigeminal artery (arrowhead).
Prior to the procedure, the patient had signed a consent form approved by the institutional review board to participate in a company-sponsored clinical trial (FDA, I.D.E.G950195, Target Therapeutics, Fremont, CA) of detachable silicone balloons for the treatment of CCFs. After the diagnostic angiogram, 8F sheaths were placed in both common femoral arteries, through which two 8F guiding catheters were placed in the right internal carotid artery. Baseline activated coagulation time was checked, and the patient received 5000 U of heparin for anticoagulation.
Initially, a 1505M detachable silicon balloon was mounted on a 0.5-cm extended-tip Tracker catheter (Target Therapeutics), purged of air by means of a vent mandril (Target Therapeutics), and advanced through one of the sheaths. A 1505 nondetachable balloon (Target Therapeutics) was similarly prepped and advanced into the carotid via the second sheath. Initial efforts to place the detachable balloon across the defect in the carotid and into the cavernous sinus by simply inflating and deflating the device were unsuccessful; this atypically resulted in the balloon sailing cephalad with the normal carotid flow. Ultimately, the balloon was minimally inflated at the orifice of the fistula while the second nondetachable balloon was positioned alongside and fully inflated, and the detachable device was pushed into the cavernous sinus. Further inflation of the detachable balloon with 0.2 mL of iso-osmolar iodinated contrast material sealed the fistula, and this was confirmed by angiography performed through the guiding catheter (Fig 2).
A and B, Lateral (A) and anteroposterior (B) views of the right internal carotid artery injected after initial placement of a detachable silicone balloon through the rent in the internal carotid artery. This placement is facilitated by the use of a second, nondetachable balloon.
C, Lateral view of the left vertebral artery injection shows the balloon catheter in place (arrowhead denotes the platinum tip of the catheter) with absence of filling of the persistent trigeminal artery.
The nondetachable silicon balloon and its guiding catheter were temporarily exchanged for a diagnostic catheter, which was used to obtain a vertebrobasilar angiogram, which also documented closure of the fistula with preservation of basilar artery flow (Fig 2). Subsequently, the nondetachable balloon and its guide were repositioned in the right internal carotid artery, where a nondetachable “safety” balloon was positioned within the C5 carotid (directly across the tip marker of the detachable balloon) (Fig 3A). At this time, the safety balloon was fully inflated within the lumen of the carotid and gentle traction was placed on the detachable balloon catheter, resulting in detachment without migration of the balloon into the cavernous carotid (Fig 3B). The nondetachable balloon was deflated, and the detached balloon remained stable under fluoroscopic observation for several minutes. Repeat angiography confirmed complete fistula closure with preservation of the parent vessels. Anticoagulation was reversed with protamine, and the patient was admitted overnight. The next day she remained free of an audible bruit with improved vision, proptosis, and chemosis. Follow-up at 6 and 12 months showed continued improvement with resolution of all symptoms, normalization of ocular pressure and visual acuity, and stable position of the inflated detachable balloon on all follow-up imaging studies.
A, Detachable silicone balloon securely placed (open arrow) just prior to inflation of a nondetachable balloon (arrowheads) precludes migration of detachable balloon into the carotid.
B, Diagrammatic representation of placement of the detachable balloon within the cavernous sinus, followed by inflation and sealing of the fistula with positioning of the nondetachable balloon at the defect in the vessel. Finally, the balloon is detached only after inflation of the nondetachable balloon within the carotid, effectively precluding migration and errant embolization of the detachable balloon into the internal carotid artery.
Discussion
A direct CCF presents as a classic clinical syndrome of sudden-onset proptosis, chemosis, bruit, and visual disturbance, most often in the setting of head trauma, cavernous sinus aneurysm, fibromuscular disease, or connective tissue disorders and/or dissection (8). Indications for relatively urgent treatment include rapid loss of vision, the presence of a large pseudoaneurysm or cavernous sinus varix (risk of subarachnoid hemorrhage), thrombosis of venous outflow tracts, and the presence of cortical venous drainage (risk of venous infarction) (9).
The case described herein represents a relatively unusual instance of CCF in association with a persistent trigeminal artery, presumably related to aneurysm rupture near the carotid origin of the vessel (10). According to McKenzie et al (11), the posterior circulation anatomy associated with this lesion may be variable; in this case, it provided a challenge to initial placement of the balloon into the cavernous sinus.
As noted by Tomsick et al (12), implementing the device consists of advancing the catheter manually under radiographic control, “. . . its passage aided by alternately inflating and deflating the balloon while gently pushing it ahead to traverse with flow the cervical and petrous ICA up to the level of the fistula. Balloon passage through the ostium is frequently seen as a sharp deflection of balloon course, or vibratory movement in the turbulence of the fistula flow. The balloon will easily pass through a high-flow fistula, but a smaller fistula may require more manipulation. . . .” According to Guglielmi et al (10), in this particular situation, balloon placement may be facilitated by a second, nondetachable balloon that is used to push the device into the cavernous sinus.
For our patient, we used a second, nondetachable balloon solely for the purpose of stabilizing placement of the first device at the time of detachment (regardless of the configuration of the CCF). By placing and then deflating the implantable balloon in the sinus, the safety balloon can likewise be directed to the defect in the carotid by the fistulous flow. Once at the rent in the carotid, it, too, can be deflated and attention turned to sealing the fistula by inflating the detachable balloon in the sinus, using angiographic control. The safety balloon is then maximally reinflated within the carotid such that at the time that traction is placed on the implantable device it is impossible for it to migrate to the arterial circulation. This practice is somewhat analogous to that described by Sanders et al (13) for the deployment of endovascular coils in the treatment of intracranial aneurysms, although we chose to use this method routinely for the detachment of silicone balloons in the cavernous sinus.
Conclusion
The method described herein reduces the well-described risk of catastrophic migration and embolization of detachable balloons into the intracranial circulation, resulting in serious cerebral infarction (7). Other risks, such as thromboembolic stroke, balloon rupture, or premature deflation (with resultant pseudoaneurysm or recurrent fistula), may relate to less generic differences in the design and material of the balloon shell as well as the valve, the inflating agent, and the method of delivery (7). These differences have been well described in previous reports and do not preclude the use of a second safety balloon for detachment.
Footnotes
↵1 Address reprint requests to Thomas J. Masaryk, MD, Department of Radiology / Hb 6, The Cleveland Clinic, 9500 Euclid Ave, Cleveland, OH 44195.
References
- Received October 8, 1998.
- Accepted after revision January 11, 1999.
- Copyright © American Society of Neuroradiology