Abstract
BACKGROUND AND PURPOSE: Few reports have documented signal abnormalities within the corpus callosum on MR studies obtained after ventricular decompression in patients with hydrocephalus. Our purpose was to establish the frequency of this finding in shunted patients and attempt to elucidate its cause and clinical significance.
METHODS: All patients with hydrocephalus shunted between 1989 and 1999 with postoperative MR studies available for review were included in the study group. Imaging analysis consisted of documenting hypointense signal on T1-weighted sagittal images and hyperintense signal on double-echo T2-weighted axial images within the corpus callosum.
RESULTS: Characteristic signal abnormalities in the corpus callosum were noted in nine of 161 patients with shunted hydrocephalus studied with MR imaging. All nine patients were asymptomatic in regard to these MR findings. Comparison with preoperative scans and surgical records revealed that all patients with signal changes on postshunt scans had chronic obstructive hydrocephalus at presentation. Preshunt MR images were notable for marked elevation of the corpus callosum, which subsequently descended after ventricular decompression, suggesting that the cause of the signal changes was related to compression of the corpus callosum against the rigid falx.
CONCLUSION: Signal abnormalities within the corpus callosum after ventricular shunting for obstructive hydrocephalus are not uncommon and are probably produced by compression of the corpus callosum against the falx before ventricular decompression. This distinctive appearance should not be mistaken for significant disease. Recognition of this pattern of signal abnormality will help avoid unnecessary intervention.
Changes in the appearance of the corpus callosum have been reported previously in patients with hydrocephalus after ventricular shunting on both CT and MR studies (1–3). Several causes have been suggested, including edema, ischemia, and demyelination. Most authors have implicated long-standing compression of the corpus callosum against the rigid falx as the causative agent. We undertook this retrospective study to determine the frequency of this phenomenon in shunted patients and to investigate its relationship to obstructive versus communicating forms of hydrocephalus.
Methods
A total of 676 patients underwent ventriculoperitoneal shunting at our institution between 1989 and 1999. Of these, 206 patients had at least one postoperative MR examination after the shunt procedure. MR studies were successfully retrieved in 176 cases for this retrospective review. Fifteen cases were subsequently excluded because of a variety of conditions that precluded adequate assessment of the corpus callosum (eg, agenesis/dysgenesis, tumor infiltration, ventriculitis, etc). The remaining 161 patients constituted our study population.
All MR examinations were reviewed by one of two neuroradiologists. All studies were performed on 1.5-T superconducting MR scanners. Although protocols varied over the 10-year period, retrospective evaluation was limited to sagittal T1-weighted sequences (500/20 [TR/TE]) and axial double-echo T2-weighted sequences (2000/20,80), which were common to all studies. Signal changes characterized by hypointensity on sagittal T1-weighted sequences and hyperintensity on axial proton density– and T2-weighted sequences were recorded as present or absent. The geographic location of signal abnormality within the corpus callosum was documented as involving one or more of the following regions: genu, anterior portion of the body of the corpus callosum, posterior portion of the body of the corpus callosum, and splenium. Preoperative MR examinations, when available, were also reviewed in all patients with positive callosal findings, and the callosal height on both examinations was recorded. Callosal height was calculated by measuring the distance from baseline (a line drawn tangential to the inferior margins of the rostrum and splenium) to the superior margin of the body of the corpus callosum as defined by Hofmann et al (4). Complete neurosurgical chart reviews were performed in all patients with abnormal callosal signal.
Results
In the study population, 109 patients had been treated for obstructive hydrocephalus and 52 for communicating hydrocephalus. Callosal signal changes were observed in 23 patients. Thirteen of these 23 studies showed small clefts of abnormal signal within the body of the corpus callosum adjacent to the shunt catheter (Fig 1) that were most likely iatrogenic. An additional case of a small cavitary lesion in the splenium in proximity to the shunt catheter was also observed and was similarly attributed to iatrogenic injury. Nine (5.6%) of all shunted patients, or 8.3% of shunted patients with obstructive hydrocephalus, exhibited more extensive signal changes of the corpus callosum, predominantly involving the anterior and posterior portions of the callosal body (patients 2 and 3). All nine presented with marked, obstructive forms of hydrocephalus attributable to obstruction at the level of the aqueduct of Sylvius (Table). Duration of symptoms of increased intracranial pressure varied from 6 weeks to 3 years (mean, 1 year). Corpus callosal height ranged from 4.0 to 4.8 cm (average, 4.3 cm) on the preshunt scans and from 1.8 to 3.4 cm (average, 2.5 cm) on the postshunt scans. This compares with an average height of 2.3 cm in a control population (4). The interval between shunting and follow-up MR evaluation ranged from 3 to 75 months (Table).
Subgroup of shunted patients with corpus callosal signal changes
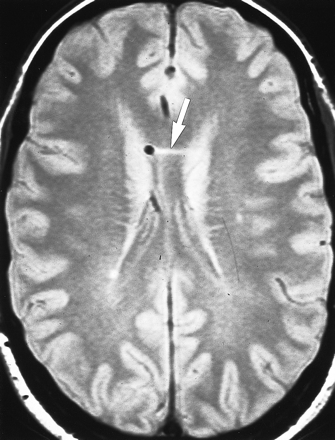
Iatrogenic callosal injury. Axial proton density–weighted (2200/20/1) image shows small horizontal cleft of increased signal within the body of the corpus callosum (arrow) adjacent to the shunt catheter that is probably related to direct trauma sustained during catheter placement
Discussion
An abnormal appearance of the corpus callosum on CT and MR studies in patients after ventriculoperitoneal shunting has been described previously (1–3). Numaguchi et al (1) described morphologic changes in the corpus callosum after ventricular shunting in six of 35 patients. In three of the six patients, scalloping of the dorsal surface of the corpus callosum was associated with “localized decreased signal” on sagittal T1-weighted images. Scalloping was attributed to ventral collapse of the corpus callosum after shunt placement with segmental tethering of the dorsal surface at sites where arterial rami of the pericallosal artery perforate the body of the corpus callosum. These changes were noted in patients treated for both communicating and obstructive hydrocephalus; however, four of the six were of the obstructive type, with tumors of the tectum obstructing the aqueduct. These authors hypothesized that the signal changes were secondary to “softening” of the corpus callosum as a result of stretching associated with long-standing hydrocephalus. Spreer et al (2) noted “hypodensities in the anterior part of the corpus callosum” on CT scans in seven of 79 patients after ventriculoperitoneal shunting. The most common causative factor in their series was aqueductal stenosis. They associated the presence of callosal hypodensity with “forced ventricular drainage,” noting that all seven patients had extreme narrowing of the ventricles and/or subdural hygromas. They invoked the theory of Numaguchi et al (1) that callosal scalloping was indicative of tethering by branches of the pericallosal artery but furthermore contended that this tethering, exacerbated by overshunting, caused a degree of traction upon these callosal perforators sufficient to induce postshunt ischemia. Both Numaguchi et al (1) and Spreer et al (2) noted that in a few of their cases, the findings were less apparent on follow-up studies and suggested that these findings were potentially reversible owing to the “gradual resorption of CSF from the callosal sulcus and reconstitution of the internal structures of the corpus callosum.”
Suh et al (3) reported two cases in which signal changes were noted diffusely throughout the body of the corpus callosum on MR studies obtained after ventriculoperitoneal shunting. No symptoms were referable to these changes, despite extensive neuropsychological testing. Both cases involved patients with aqueductal obstruction and moderate to marked degrees of hydrocephalus. The published images bear a striking resemblance to the cases encountered in our series.
We did not observe any of these signal changes in patients with communicating forms of hydrocephalus. Additionally, all patients in this series with callosal signal changes had obstruction at the level of the aqueduct. This correlation with aqueductal obstruction is noteworthy. Hofmann et al (4) showed that elevation of the body of the corpus callosum in patients with aqueductal obstruction is significantly greater than that in patients with communicating forms of hydrocephalus. We were able to demonstrate significantly increased corpus callosal height in eight of nine patients with callosal signal changes; no preoperative studies were available in case 6 (Table). This increase in dorsal bowing of the callosal body in obstructive hydrocephalus probably predisposes it to compressive injury against the undersurface of the rigid falx more so than communicating forms of hydrocephalus, with less significant degrees of elevation in callosal height. It would stand to reason that the elevated intraventricular pressures present in patients with obstructive hydrocephalus would be expected to produce a greater degree of compression than might be encountered in patients with communicating forms of hydrocephalus. This long-standing compression probably compromises the venous drainage or even arterial supply to the corpus callosum, leading to the signal changes. Spreer et al (2) excluded this as a mechanism of injury owing to a lack of findings on preshunt studies; however, it was our observation that adequate assessment of signal changes within the corpus callosum on preoperative examinations was not possible because of the severe stretching and attenuation of this structure. It was only after the ventricles had been decompressed and the corpus callosum had reexpanded that the findings became obvious (Figs 2A and B and 3A and B). We consider it highly unlikely that the signal changes are secondary to traction-induced arterial compromise, as proposed by Spreer et al (2), since we were unable to document any evolution in appearance over time, as might be expected if these signal changes were produced by ischemia alone. Several of the nine patients in our series with typical callosal signal had multiple follow-up studies with no significant change in appearance. Although we did find significant degrees of ventricular decompression on the postshunt scans, as evidenced by the reduction in corpus callosal height (Table), no association between overshunting or “forced ventricular drainage” and callosal changes as reported by Spreer et al (2) were found in our series.
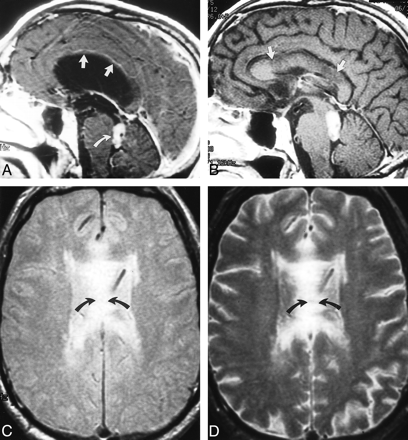
Case 7: callosal injury from long-standing obstructive hydrocephalus.
A, Sagittal T1-weighted (500/15/2) image obtained before shunt placement shows marked upward displacement of the corpus callosum (straight arrows). Note fourth ventricular mass (curved arrow) extending into and obstructing the aqueduct.
B, Sagittal T1-weighted (500/20/2) image obtained 3 months after ventricular shunting shows diffuse areas of decreased signal within the body of the corpus callosum (arrows), which is likely the result of compression against the falx.
C and D, Axial proton density– (C) and T2-weighted (D) (2200/20,80/1) images show increased signal within the body of the corpus callosum (arrows), corresponding to abnormalities in the sagittal plane.
The mechanism of compression of the corpus callosum against the falx in patients with significant hydrocephalus as a cause of callosal injury has been proposed previously. Jinkins (5) noted localized dorsal flattening and thinning of the posterior body of the corpus callosum in 24 of 40 patients with untreated communicating hydrocephalus. Unfortunately, none of these patients had undergone shunting at the time of publication. Because the falx is anatomically more complete posteriorly, localized compression of the dorsal aspect of the falx would be expected. Suh et al (3), however, noted a more diffuse pattern of injury encompassing the entire body of the corpus callosum in his two patients and proposed a more diffuse compressive injury. The distribution of signal changes in our series of patients would support a more extensive injury (Table). The two patients in the study by Suh et al (3) and the nine patients in our series all had moderate to marked obstructive hydrocephalus, and we would therefore expect more extensive injury to the corpus callosum than that seen in patients with communicating hydrocephalus. Even among patients with similar degrees of severe obstructive hydrocephalus, some variability in the extent of injury would be expected given the natural variation in the height of the falx. Galligioni et al (6) found as much as a 2-cm variation in height in their study of 200 cerebral arteriograms. For this reason, the degree of elevation of the corpus callosum in hydrocephalus may not always correlate with the degree of corpus callosum injury, depending on the anatomic height of the falx in a given individual. We did not confirm the observation by Spreer et al (2) that callosal changes are most commonly seen in the “anterior part of the corpus callosum.” The signal changes in our series uniformly involved both the anterior and posterior portions of the body of the corpus callosum. Two of the nine patients in our series had signal abnormalities in the genu, but only in addition to extensive involvement of the body of the corpus callosum. It is likely that the patients in the series published by Spreer et al (2) also had involvement of the body of the corpus callosum that could not be adequately seen on CT scans owing to the large slice thickness (8 mm) and problems associated with volume averaging with adjacent CSF.
The duration of symptoms leading to the diagnosis of obstructive hydrocephalus averaged about 1 year in our series of patients. It is likely that callosal injuries of this type produced by compression against the rigid falx are more apt to be encountered in instances of long-standing, unrelieved compression. Several patients in our study presented with severe, acute obstructive hydrocephalus from intraventricular hemorrhage, and none of them manifested any signal abnormalities within the corpus callosum on the postshunt examinations.
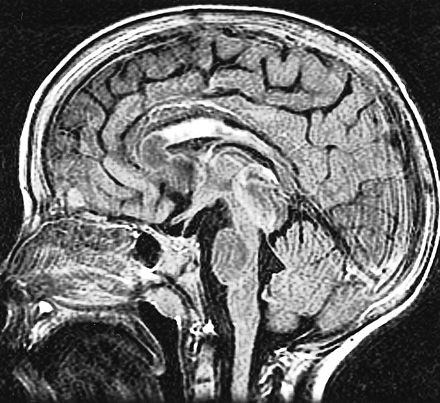
Only spin-echo sequences were included in this retrospective review, because these sequences were common to all examinations dating back 10 years. Several of our more recent cases included fluid-attenuated inversion-recovery (FLAIR) sequences in the imaging evaluation, and these were superior to the spin-echo sequences in showing the callosal injury. In particular, we have found that FLAIR sagittal sequences characterize this signal abnormality to the greatest advantage (Fig 4).
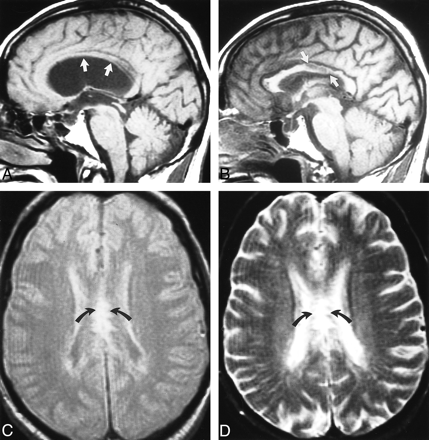
Case 2: callosal injury from long-standing obstructive hydrocephalus in a patient with aqueductal stenosis.
A, Sagittal T1-weighted (500/15/2) image obtained before shunt placement shows moderate upward displacement of the corpus callosum (arrows).
B, T1-weighted sagittal (500/15/2) image 5 months after ventricular shunting shows diffuse areas of decreased signal within the posterior body of the corpus callosum (arrows), which was most likely caused by compression against the falx.
C and D, Axial proton density– and T2-weighted (2200/20,80/1) images show increased signal within the body of the corpus callosum (arrows) corresponding to abnormalities in the sagittal plane.
Case 1: callosal injury in a patient with obstructing tectal tumor. Postshunt sagittal FLAIR sequence (11002/148/1, TI = 2250) proved to be the most sensitive in detecting callosal signal changes but was not included in the imaging protocols of all examinations reviewed
Results of the chart reviews in our nine patients with corpus callosum injury revealed no clinical symptoms associated with these findings. Although neuropsychological testing was not performed, we concur with Suh et al (3) that these findings are probably of no clinical significance. The value in recognizing this distinctive pattern on diagnostic imaging is in avoiding misinterpretation of these findings as significant disease, such as infiltration from neoplasms that commonly cross the corpus callosum (eg, lymphoma or glioblastoma) and demyelinating disorders such as multiple sclerosis, osmotic demyelination, or progressive multifocal leukoencephalopathy.
Conclusion
A characteristic pattern of signal alteration within the body of the corpus callosum can occasionally be seen in patients scanned after shunting for obstructive hydrocephalus. The cause of injury to the corpus callosum in these cases is most likely related to a relatively long-standing compression of the fibers of the corpus callosum against the undersurface of the falx. This injury does not appear to produce any clinically recognizable symptomatology. Recognition of this distinctive appearance is helpful to avoid misinterpreting these findings as significant disease, which may lead to unnecessary intervention.
Footnotes
↵1 Address reprint requests to John I. Lane, MD, Department of Diagnostic Radiology, Mayo Clinic, CH2–213; 200 First St SW, Rochester, MN 55905.
- Received February 7, 2000.
- Copyright © American Society of Neuroradiology