Abstract
BACKGROUND AND PURPOSE: Preliminary data indicate that apparent diffusion coefficient (ADC) values may be useful in identifying and grading primary cerebral tumors. We tested the hypothesis that ADC values can be used to differentiate tumor, edema, and normal brain tissue.
METHODS: Fifteen patients with high-grade cerebral astrocytomas underwent conventional MR imaging, diffusion-weighted MR imaging, and proton MR spectroscopy. We defined tumor as an area containing the highest choline/creatine and choline/N-actetyl aspartate ratios, contrast enhancement, and abnormal T2 signal intensity. Edema was defined as tissue with normal proton MR spectra, no enhancement, and high T2 signal intensity. Normal brain was assumed if tissue had normal proton MR spectra, no enhancement, and normal T2 signal intensity in the hemispheres ipsilateral or contralateral to tumor. ADC maps were calculated and regions of interest were manually placed over areas of tumor, edema, and normal tissue. Comparisons were made by analysis of variance. For post hoc testing, the Tukey method was used to correct for the effect of multiple comparisons, and significance was accepted if P was less than .05.
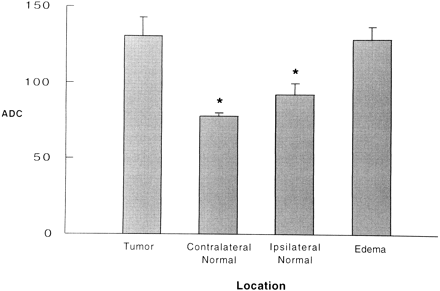
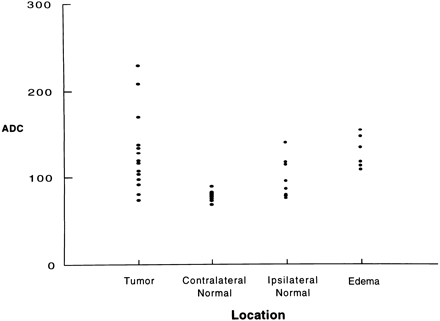
RESULTS:When ADC values were analyzed as a group, significant differences were found between tumor (131 + 45) and normal brain tissue (ipsilateral to tumor, 92 + 22; contralateral to tumor, 78 + 5) but not between tumor and adjacent edema (129 + 45). A plot of individual data points showed considerable overlapping among the three types of tissue sampled.
CONCLUSION: As a group, ADC values helped to distinguish high-grade glioma from normal tissue but could not be used to separate high-grade glioma from surrounding edema. Individually, ADC values overlapped considerably and were not useful in our patients. The utility of ADC values (as obtained in this relatively small study) is questionable in patients with high-grade cerebral astrocytomas.
Because the correlation between conventional MR imaging findings and histologic tumor demarcation is imperfect, there is a need for other imaging techniques capable of defining tumor boundaries. Proton MR spectroscopy may provide this information but requires small voxels and at least a 2D acquisition. Echo-planar diffusion-weighted MR imaging is rapidly performed and has become a mainstay in the examination of patients with stroke. Recent articles suggest that values obtained from apparent diffusion coefficient (ADC) maps may play a role in the evaluation of tumors (1-5). Characterization of tumors by ADC values may not only help to target the most malignant area of highest cellularity, but also to demarcate tumors from surrounding tissue and to identify recurrent or residual malignancy. We sought to determine whether ADC values are more useful than conventional MR images in differentiating tumor, edema, and normal brain tissue.
Methods
Conventional contrast-enhanced MR imaging, ADC mapping, and proton MR spectroscopy were performed in 15 patients (three women and 12 men, 35-75 years old) with malignant cerebral astrocytomas. Conventional MR imaging was performed on 1.5-T MR units with a protocol that included sagittal noncontrast T1-weighted sequences (560/15/1 [TR/TE/excitations]), axial fast spin-echo T2-weighted sequences (4500/15,45/1), and contrast-enhanced axial, coronal, and sagittal T1-weighted sequences using the same parameters as the noncontrast studies. ADC maps were acquired (before administration of contrast material) using b values of 0, 500, and 1000 s/mm2 applied in the z, y, and x directions. Postprocessing of ADC maps was performed off-line by using an independent computer workstation. All proton MR spectroscopy studies were done using a 2D technique and water suppression with chemical shift-selective excitations; the volumes were localized using point-resolved spectroscopy with parameters of 1500/270/128-256. The grid was placed over the center of the enhancing region but also included areas of edema and normal-appearing brain. All voxels were manually postprocessed and baseline and phase-shift corrected. Individual volumes varied in size from 1 × 1 × 1cm to 1.5 × 1.5 × 1.5 cm for different patients according to the area of coverage required. We assigned the following resonances: N-acetylaspartate (NAA) at 2.0 parts per million (ppm), choline (Cho) at 3.2 ppm, creatine (Cr) at 3.03 ppm, and lipids and lactate at 1.2 to 1.5 ppm. Using the curve-fitting software provided by the MR unit manufacturer, we obtained areas for the peaks of NAA, Cho, and Cr, and calculated ratios of Cho/Cr and Cho/NAA. All proton MR spectra were obtained before administration of contrast material or on the day after the contrast-enhanced MR study.
For each lesion, we defined the following regions on the basis of imaging features: 1) tumor, as an area containing the highest Cho/Cr and Cho/NAA ratios, contrast enhancement, and abnormal T2 signal intensity; 2) edema, as an area containing normal Cho/Cr and Cho/NAA ratios, no enhancement, and high T2 signal intensity; and 3) normal tissue, as an area containing normal Cho/Cr and Cho/NAA ratios, no enhancement, and normal T2 signal intensity in the hemisphere ipsilateral (n=11) or contralateral (n=14) to tumor. We then manually selected a region of interest (ROI) on the ADC maps corresponding as closely as possible in location and size to the three (or four) regions previously described. Comparison among groups was done using analysis of variance. Post hoc testing used the Tukey method to correct for the effect of multiple comparisons. A significant difference was accepted if P was less than .05. Histologic confirmation as to the exact nature of the tissues sampled was not obtained.
Results
Four patients had newly diagnosed tumors and 11 had recurrent/residual tumors (all had undergone surgery and radiation therapy or chemotherapy or both). Biopsies were performed in all tumors: eight were glioblastoma multiforme (GBM) and six were anaplastic astrocytoma. We then compared the ADC values in three (or four, if measurements for the contralateral hemisphere were available) ROIs (tumor, edema, and normal brain) for each patient (Fig 1). When ADC values were analyzed in a group fashion, significant differences were found between tumor (131 + 45) and normal tissue ipsilateral, or contralateral to tumor (92 + 22 and 78 + 5, respectively) and edema (129 + 45) and normal tissue ipsilateral or contralateral to tumor (Fig 2). When individual data points were plotted, considerable overlapping was present among all groups (Fig 3).
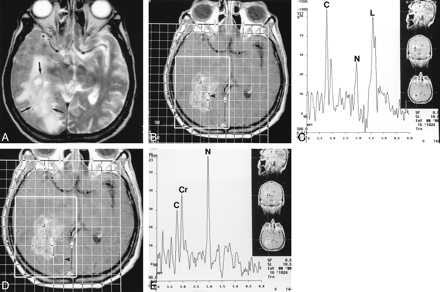
GBM after radiation/chemotherapy.
A, Axial T2-weighted image shows a mass in the white matter (arrows) of the right occipital lobe, with surrounding edema extending into the temporal region. The mass is of heterogeneous signal intensity.
B, Corresponding axial contrast-enhanced T1-weighted image with superimposed grid shows position of voxel (arrowhead) with highest Cho/Cr and Cho/NAA rations.
C, Corresponding proton MR spectrum shows marked elevation of Cho (C), low NAA (N), and a large peak that probably corresponds to lactate (L).
D, Same contrast-enhanced image as in B shows position of voxel (arrowhead) identified as containing edema (compare with A).
E, Proton MR spectrum from voxel selected in D shows normal metabolites (choline = C, creatine = CR, and NAA = N).
F, ADC map shows position of ROIs corresponding to tumor (1) and edema (2). Values are 103.4 + 10.6 and 153.7 + 14.4 for tumor and edema, respectively.
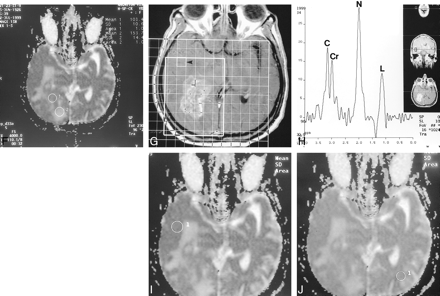
Continued.
G, Axial contrast-enhanced T1-weighted image with grid shows position of voxel (arrowhead) representing normal tissue.
H, Corresponding proton MR spectrum shows normal Cho (C), Cr, and NAA (N); lactate (L) is elevated as a result of therapy.
I, ADC map with ROI corresponding to area in G (1). ADC is 84.4 + 6.9.
J, ADC map with ROI (1) in normal brain tissue in hemisphere contralateral to tumor. ADC is 83.6 + 6.6.
Bar graph of ADC values (s/mm2) for tumor, contralateral normal tissue, ipsilateral normal tissue, and edema for the group of 15 patients with high-grade cerebral gliomas. Note difference between tumor and normal brain but lack of difference between tumor and edema. * = statistically significant
Plot of individual data points for tumor contralateral normal tissue, ipsilateral normal tissue, and edema. Note considerable overlap of all points
Discussion
Diffusion-weighted MR imaging is easily performed using echo-planar-capable units, and, because it is sensitive to alterations in the tranlational (brownian) motion of water molecules in the region of the brain studied, it has had a significant impact on clinical imaging. Diffusion-weighted MR imaging is used primarily for the evaluation of stroke but has also been used to assess brain infections and tumors. In one study using ADC maps (applying the diffusion-sensitizing gradient in only one direction), the authors stated that they were able to differentiate tumor, necrosis, cysts, and edema, and concluded that the technique was a “powerful tool in the characterization of brain neoplasms” (1). Detailed analysis of the data presented presented in that publication reveals that no significant differences were found in the ADC values of edema in white matter parallel to the diffusion-sensitizing gradient, in parallel white matter, and in nonenhancing tumor in parallel white matter. Enhancing tumor had the lowest ADC values and cyst/necrotic tumor the highest. Thus, ADC values were helpful in distinguishing between tumor or edema and nonedematous, nontumorous tissues. In experimental studies, quantitative ADC maps were calculated for three different types of implanted brain tumors in rats (2, 3). Although the ADC maps were obtained using only one b value, the authors concluded that ADC values could not be used to differentiate tumor types and that, even if tumor could be distinguished from edematous tissue and normal brain, a sharp demarcation between tumor and edema was not possible. They attributed this observation to an enlargement of the extracellular compartment, which occurs as fluid spreads from the edge of the tumor to the surrounding tissues, most likely canceling the effect on increased cellularity (lower ADC) and vasogenic edema (higher ADC).
ADC values have also been correlated with the degree of tumor cellularity. Because the majority of the translational movement of water occurs in the extracellular space, swelling or increased cellularity should affect the ADC. Thus, the lowest ADC value should indicate the region of greatest cellularity and be helpful in selecting biopsy targets. In one study of 20 patients with astrocytomas of different grades from whom ADC maps were obtained, increasing tumor cellularity corresponded to lower ADC values but tumor cellularity and T2 signal intensity showed no correlation (4) Thus, GBM had the lowest ADC; anaplastic astrocytoma, an intermediate ADC; and low-grade astrocytoma, the highest ADC.
Proton MR spectroscopy findings seem to indicate that there is a correlation between ADC and levels of Cho in tumors. Gupta et al (5) studied 20 tumors with proton MR spectroscopy and ADC maps and found that areas of dense hypercellular tumor showed increased Cho and low ADC. Increasing tumor cellularity with decreasing extracellular space creates an environment in which water motion is restricted outside the cells and in the more viscous and complex intracellular space. Gupta et al (5) found some cases in which ADC was high in regions of elevated Cho, a finding they attributed to the presence of microcysts and interstitial edema. These authors concluded that statistically significant support was found for the interpretation of elevated Cho signal intensity in terms of tumor cellularity and proliferative potential as seen on ADC maps. The importance of this study lies in the discerned correlation between ADC and Cho levels in tumors.
Because of the varying results reported in the literature, we decided to evaluate 15 high-grade astrocytomas with ADC mapping and proton MR spectroscopy. We did not seek to establish which technique is best but solely to test the hypothesis that ADC values can differentiate tumor, edema, and normal brain. In addition, we did not seek to establish the utility of ADC in tumor grading. If ADC values prove useful in tumor identification, the next logical step would be to generate tumor maps based on diffusion-weighted imaging, which would facilitate surgery and follow-up of these patients. Because many of our patients had undergone prior surgery and were not amenable to further resection and tumor sampling, we did not obtain histologic samples of each ROI; instead, we chose to define three different tissues sampled according to their imaging and proton MR spectroscopy characteristics. Contrary to other studies (1, 4), we applied the diffusion-sensitizing gradient in three directions, thus potentially increasing the sensitivity of the ADC maps. By manually positioning the ROIs on the ADC maps corresponding to the imaging/proton MR spectroscopy-defined tissues, we avoided macroscopic necrotic and cystic-appearing areas. The use of echo-planar diffusion-weighted imaging negates most of the contribution from bulk motion, and our ADC values are most likely a reflection of mainly microscopic water motion.
We did not perform a pixel-by-pixel analysis of ADC maps because of technical restrictions and, despite slight differences in the size and shape of ADC ROIs and proton MR spectroscopy voxels, we do not think this affected our results in any significant way. Similar to earlier studies, we found a difference between tumor, edema, and normal brain when results were compared as groups but not when they were compared as individual data points. Thus the utility of ADC maps in the evaluation of individual brain tumors is questionable. For practical purposes, we grouped patients with anaplastic astrocytoma and GBM together. We cannot completely exclude the possibility that microscopic necrosis influences our data somewhat, but all ADC values were higher among patients with anaplastic glioma than in those with GBM. We found a restriction of translational water motion in edematous and neoplastic tissues. We speculate that in white matter edema (vasogenic) there is more water in the extracellular space, leading to increased random motion and lower ADC. The low ADC values in tumor probably reflect a decreased volume of extracellular space due to encroachment by tumor cells and increased intracellular viscosity, with subsequent restriction of water motion.
Diffusion measurements have also been used to study tumor response to treatment. In an animal model of glioma, tumors that were treated with chemotherapy showed increasing ADC values in the 6 to 8 days after treatment, probably corresponding to acute necrosis and a loosening of the extracellular space (6). In the presence of tumor regrowth, ADC values again dropped and returned to pretreatment levels. Therefore, ADC maps may have some utility for monitoring the response to tumor treatment-an observation that needs to be tested in humans.
Conclusion
In our findings in a relatively small group of patients with high-grade gliomas, ADC values were of little clinical utility in outlining the tumor with respect to edematous surrounding tissues. Although we were able to identify differences in ADC values between high-grade gliomas and edema/normal brain when our patients were compared as groups, individual data plotting showed considerable overlap among high-grade gliomas, edema, and normal brain. ADC values were not significantly different between edema and normal brain.
Footnotes
1 Address reprint requests to Mauricio Castillo, MD.
- Received January 7, 2000.
- Copyright © American Society of Neuroradiology