Abstract
Summary: We describe a case of a 63-year-old man with chronic-contained rupture of an abdominal aortic aneurysm at the site of prior graft repair of the aneurysm. Initially misinterpreted as osteomyelitis on the basis of CT findings, this chronic-contained rupture of the abdominal aorta eroding the vertebrae was preoperatively diagnosed at MR imaging and confirmed at surgery. A conventional angiogram failed to show the pseudoaneurysm. Owing to a major difference in the management of a contained aortic aneurysm rupture versus that for osteomyelitis, MR imaging with CT or MR angiography is recommended before any operative or invasive procedure.
Choplin et al (1) described a ruptured abdominal aortic aneurysm simulating pyogenic vertebral spondylitis in 1982. Various investigators (2, 3) described chronic-contained rupture of abdominal aortic aneurysm as early as 1986. Our review of seven cases (1, 4–7) of chronic-contained rupture of an abdominal aortic aneurysm with vertebral erosion revealed that the most common presenting symptom in hemodynamically stable patients was back pain. MR imaging was performed in only one patient to confirm the diagnosis (5). In this report, we describe the case of a 63-year-old man with low backache 2 years after the repair of an abdominal aortic aneurysm. MR imaging helped differentiate the cause of the vertebral destruction as chronic-contained abdominal aortic aneurysm rupture rather than osteomyelitis.
Case Report
A 63-year-old Caucasian man presented with low back pain 2 years after the repair of an abdominal aortic aneurysm with a graft. CT scans of the abdomen showed lysis and destruction of the second and third lumbar vertebrae (Fig 1A and B), and the intervening disk as a hypoattenuating, nonenhancing, soft-tissue mass measuring 8.0 × 6.0 cm. The margins of the vertebral lesions were well corticated. The erosion extended to the posterior third of the second lumbar vertebral body and through the cortex of the posterior margin of the third lumbar vertebral body, with extension to the epidural space. No narrowing of the spinal canal was present; however, the mass had a hypoattenuating center that suggested fluid content, and it displaced the aorta anteriorly as it extended into the paravertebral space and left psoas muscle. On several images, the posterior wall of the aorta was not visualized separately from the mass. The aorta was dilated from the level of the celiac artery to its bifurcation. It measured approximately 4 cm in diameter. The CT finding was interpreted as vertebral osteomyelitis with paraspinal abscess. Subsequently, the patient underwent CT-guided aspiration of the left psoas fluid collection because of the suspicion of infection. Hemorrhagic fluid was obtained; no pathologic organisms grew on cultures of this fluid. Despite the negative culture finding, the patient was given a course of antibiotics.
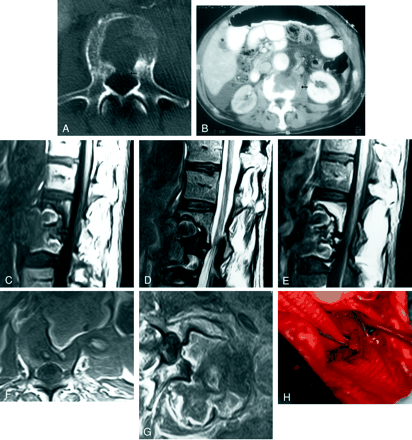
Images in a 63-year-old Caucasian man with low back pain 2 years after the repair of an abdominal aortic aneurysm with a graft.
A, Axial CT image of the third lumbar vertebra shows a large lytic defect in its body with a cortical discontinuity along the posterior margin (arrow) that suggests epidural extension.
B, Axial contrast-enhanced CT image of the abdomen shows a hypoattenuating mass in the third lumbar vertebral body, with left paravertebral soft-tissue extension (arrow). The aorta is enhancing normally, with a crescent of normal peritoneal fat along the left lateral margin. The kidneys are enhancing asymmetrically because of a right renal artery stenosis at the anastomotic site.
C–E, Sagittal T1-weighted (655/13/2) (C), sagittal T2-weighted (4000/100/2) (D), and sagittal contrast-enhanced T1-weighted (784/14/1) (E) MR images of the lumbar spine show a large defect in the second and third lumbar vertebrae. The anterior margins of the vertebrae are completely eroded by what appears to be a predominantly prevertebral mass showing minimal enhancement. The posterior margin of the aorta is not well depicted on these images.
F and G, Axial T1-weighted (655/13/2) (F) and axial contrast-enhanced T1-weighted (784/14/1) (G) MR images of the third lumbar vertebra show a large mass in the prevertebral and left paravertebral space eroding the anterior margin of the vertebral body, with extension to the anterior epidural space. The mass is extending into the left psoas muscle, and anteriorly, it appears to be continuous with the aorta.
H, Dacron grafts are depicted superiorly (clamped) and inferiorly (from previous surgery) medial to the retractor, with a large defect in the posterior wall of the aorta eroding into the vertebral body (center of the photograph).
MR imaging of the spine was then performed to further characterize the lesion. Axial, sagittal, and coronal T1-weighted (655/13/2[TR/TE/excitations]; axial and sagittal T2-weighted (4000/100/2); and axial and sagittal contrast-enhanced T1-weighted (784/14/1) images were obtained. The shape and margins of the mass were as described at CT. The lesion had central isointense and peripheral hyperintense signal compared with that of muscle on T1-weighted images (Fig 1C and E–G), and it had central hyperintensity and peripheral heterogeneous signal intensity on T2-weighted images (Fig 1D). No enhancement was detected in or around the lesion (Fig 1C–G). The aorta was dilated, with no discernible plane between it and the mass. This finding was thought to be a pseudoaneurysm of the aorta eroding into the vertebrae because of its signal characteristics and shape. The patient underwent conventional angiography as part of preoperative planning. Angiograms showed a dilated aorta but no pseudoaneurysm or leak at the graft site.
Subsequently, findings from elective surgery confirmed an abdominal aortic pseudoaneurysm and an organized retroperitoneal hematoma. After aortic cross-clamping and opening of the aneurysm, a large rupture (4 × 3 cm) was discovered in the posterior wall of the aorta at the superior suture line of the graft from the previous surgery (Fig 1H). Large clots of blood were evacuated from the pre- and paravertebral regions; these clots corresponded to the collections seen at CT and MR imaging. A knitted Dacron graft was inserted, and the patient responded well postoperatively. He had no back pain at discharge. No pathologic organisms grew on the cultures of the clot evacuated during surgery.
Discussion
Hallett et al (8) reviewed graft-related complications after abdominal aortic aneurysm repair in 307 patients. Twenty-nine patients (9.4%) in this study had a graft-related complication, the most common complication being a pseudoaneurysm at the anastomotic site (3.0%). The other complications were graft thrombosis (2.0%), graft-enteric erosion or fistula or both (1.6%), graft infection (1.3%), anastomotic hemorrhage (1.3%), colon ischemia (0.7%), and atheroembolism (0.3%). Eight patients (2.6%) had complications within 30 days of surgery, and 21 patients (6.8%) had complications after 30 days. No complications were related to dilatation or rupture of the prosthetic graft material. No chronic-contained abdominal aortic aneurysm rupture eroding into the spine occurred in that series.
Rupture of an abdominal aortic aneurysm can take place in the following ways (9): 1) open rupture into the peritoneal cavity; 2) closed rupture into the retroperitoneum; 3) rupture into the surrounding hollow structures (eg, veins and bowel); and 4) chronically contained or sealed rupture, in which the leak is sealed by a surrounding inflammatory reaction. CT scans can be used to accurately distinguish abdominal or back pain attributable to a ruptured abdominal aortic aneurysm from other causes of pain in a hemodynamically stable patient. Some patients with abdominal aortic aneurysm rupture clearly demonstrable on CT scans are clinically stable for prolonged periods. Such patients are defined as having a chronic-contained rupture (also termed “sealed,” “spontaneously healed,” or “leaking”) of the abdominal aortic aneurysm (2, 3, 9–11). To our knowledge, this was first described (2, 3, 9–11) in 1986. Chronic-contained abdominal aortic aneurysm ruptures are rare. Even less common are the anecdotal reports of chronic-contained rupture and/or pseudoaneurysm causing vertebral erosions (1, 4–7).
Various causes of vertebral body masses with extension into the psoas muscle exist. The most common causes are infections and tumors (primary and metastatic). Sealed rupture of abdominal aortic aneurysm causing vertebral erosion is rare, and on review of the literature, we found seven such cases (1, 4–7). All patients were male, with a mean age of 63.4 years (range, 53–81 y). All patients were normotensive or hypertensive and hemodynamically stable at initial presentation. The most common presenting symptom was back pain (six of seven cases); the duration of symptoms ranged from 2 weeks to 2 years.
The mechanism of chronic-contained abdominal aortic aneurysm rupture is not clear. It is believed that in the case of small aneurysms, the aortic wall is still strong, and, thus, the tear is limited (11). In large aneurysms, as the sac increases in size, it incites a strong perianeurysmal reaction, which subsequently provides high resistance to extravasation of blood if the aneurysm ruptures (11). As in the current case, in a previous series, the site of the sealed abdominal aortic aneurysm rupture was posterior in 62.5% of cases (11). This finding suggests that the vertebrae can tamponade the rupture effectively because of their inherent strength (11).
These cases show the degree of bone remodeling that occurs primarily because of the contained nature of the rupture. As many as 25% of contained ruptures reveal this finding, as opposed to 2% of uncontained ruptures (7, 11). Arterial pulsation repetitively compressing the vertebrae 80–90 times per minute for a few weeks (7) to a few years may produce extensive bone destruction. Erosion of the vertebral bodies by an abdominal aortic aneurysm is seen in only 7% of cases (1). It is important to differentiate the erosion caused by an aneurysm from that caused by infection, especially in elderly patients with a history of graft repair of an abdominal aortic aneurysm. Erosion caused by aneurysms is usually smooth, in contrast to that caused by pyogenic infection in which the margins (of the lytic process) are irregular and poorly defined (3). In 15% of patients with abdominal aortic aneurysms, organisms can be cultured. Since organisms grew in cultures from all cases with chronic-contained rupture causing vertebral erosion in one other study (7), infection has been implicated as a contributing cause for this type of rupture (7). Compared with conventional angiography, cross-sectional imaging is a critical component of the evaluation of aortic aneurysms. On conventional angiograms, filling of the extraluminal component of the aortic aneurysm may not be present if the pseudosac is completely filled with thrombosed blood.
In summary, MR imaging with CT or MR angiography provides an invaluable, noninvasive means to depict the pseudosac of a chronic-contained abdominal aortic aneurysm rupture filled with blood products and to confirm its diagnosis.
Footnotes
1 Address reprint requests to Vibhu Kapoor, MD, Department of Neuroradiology, Room D-132, 200 Lothrop Street, University of Pittsburgh Medical Center, Pittsburgh, PA 15213.
References
- Received December 12, 2000.
- Accepted after revision May 18, 2001.
- Copyright © American Society of Neuroradiology