Abstract
BACKGROUND AND PURPOSE: Little is known regarding changes in the temporal lobe associated with traumatic brain injury (TBI) in early-to-mid adulthood. We report on two quantitative MR studies: study 1 addressed age-related changes of the temporal lobe in subjects aged 16–72 years; information obtained in this study provided a normative database for comparison with findings in 118 patients with TBI who were included in study 2. We expected stable morphology in healthy subjects and trauma-related atrophy in patients with TBI.
METHODS: MR multispectral tissue segmentation was used to calculate bilateral temporal lobe gyrus and sulcus, sylvian fissure CSF, hippocampus, and temporal horn volumes and to measure the white matter (WM) temporal stem.
RESULTS: With normal aging, gyral volume remained stable, decreasing approximately 0.26% per year (total, ≈11%). Sulcal CSF volume doubled. Hippocampal volume decreased (minimally, significantly); temporal horn volume increased (not significantly) and was minimally related to hippocampal volume. WM measurements were constant. Trauma changed morphology; WM measures decreased. Gyral volumes were not different between the groups. In TBI, CSF volume increased significantly, was most related to reduced WM measurements, and was relatively independent of gyral volume. Temporal horn dilatation was related more to WM atrophy than to hippocampal atrophy. In TBI, subarachnoid sulcal and temporal horn CSF volumes were most related to WM atrophy, which was relatively independent of gyral volume; gyral and hippocampal volumes and WM measures were related to memory performance.
CONCLUSION: Age-related changes cause minimal temporal lobe gyral, hippocampal, temporal horn, and WM atrophy. Only subarachnoid sulcal CSF volume changed robustly. Trauma produced disproportionate WM loss associated with increased temporal horn and sulcal CSF volumes; it caused substantial hippocampal atrophy, which was related to memory impairment. Gyral volume did not decrease, although it was related to memory performance.
Over the last decade, with the advent of semiautomated methods for image quantification, numerous articles about global measures of brain development and age-related changes obtained by quantifying findings in brain MR images have been published (1–11). The most current quantitative MR investigations have concentrated on regional differences and distinct nuclei of the brain, since this type of analysis may provide greater neuropathologic specificity for some disorders (12–19). For example, different regions of the temporal lobe are the loci of neuropathologic changes that occur in such disorders as Alzheimer disease, alcoholism, traumatic brain injury (TBI), epilepsy, depression, learning disability, stress-mediated disorders, and schizophrenia, to identify some of the more common ones (5, 12, 13, 17–19, 20–40).
The human temporal lobe is divided into five gyri and sulci. The five gyri are the superior, middle, inferior, fusiform, and parahippocampal gyri. The five sulci are the superior, middle, inferior, and rhinal sulci, with the sylvian fissure defining the CSF space dorsal to the superior temporal gyrus. The temporal horn is formed as a longitudinal and downward extension of the lateral ventricular system that is centrally located in the temporal lobe. In the floor of the temporal horn sits the hippocampus, another critical structure for cognitive function (8, 24, 41). Since hippocampal input is provided predominantly via the fusiform and parahippocampal gyri, changes in these gyri may provide additional information about the integrity of important temporal-limbic areas of the brain (19).
In the two studies included herein, quantitative MR analysis of the temporal lobe was performed in 136 healthy individuals whose ages spanned 5½ decades, beginning at age 16 years (study 1). Normative findings were compared with those obtained with similar analyses in 118 patients with TBI (study 2). Specifically, in each subject, we calculated hippocampal, temporal lobe gyral and sulcal, and temporal horn volumes and measured the white matter (WM) across the temporal stem. We selected the temporal stem WM area, because this is a region where both efferent and afferent WM pathways congregate as they enter and exit the temporal lobes. In terms of WM changes, we predicted that this area would be a convergent WM pathway that is most likely to be affected by trauma.
Considerable interest surrounds the morphologic changes of the temporal lobe that are associated with aging, particularly those that occur late in life (19, 42). However, less is known about age-related changes associated with early-to-mid adulthood, when TBI is most likely (43, 44). Accordingly, study 1 addresses age-related changes of the temporal lobe in subjects aged 16–72 years; information obtained in this study provided a normative database for comparison with findings in patients with TBI who were included in study 2.
We have previously reported a modest inverse correlation between hippocampal and temporal horn volumes (24). The assumption in neuroradiology has been that temporal horn dilatation may be a sign of hippocampal atrophy, because the hippocampus forms the ventral and medial boundaries of the temporal horn. However, the relationship between hippocampal atrophy and temporal horn expansion in aging or TBI is far from linear, and the explained variance in this relationship is minimal at best (45). In trauma, the expansion of the temporal horn could occur because of atrophic changes in gyral, lobular, or whole-brain volume. Also, the lateral surface of the temporal horn is surrounded by WM pathways, and since WM is selectively vulnerable in TBI (46), temporal horn expansion may be a reflection of loss in WM integrity. Thus, another objective of study 2 was to undertake a more detailed morphometric analysis of the temporal lobe in the context of trauma-induced hippocampal atrophy and to determine how hippocampal atrophy relates to temporal lobe gyral, temporal lobe sulcal, and temporal horn volumes and to temporal stem width in patients with TBI. On the basis of our previous findings (2, 24, 31), we hypothesized that temporal lobe structures have minimal age-related effects over this time frame, that TBI causes substantial morphologic changes, and that trauma-induced changes in sulcal and temporal horn CSF volumes are semi-independent of gyral and hippocampal volume and reflect WM damage (47).
Methods
Study 1: Temporal Lobe Morphology in Normal Aging
Subjects.—Subjects included 136 volunteers (64 male, 72 female; age range, 16–72 years; mean ±SD, 28.70 ± 7.93) who were recruited primarily from hospital and university staff and their friends and family. Most were the subjects in a previous normative quantitative neuroimaging study (2), although we extended the last group to include those whose ages spanned a decade and half (ie, subjects aged 56–72 years). Exclusion criteria included the following: 1) previous head injury causing loss of consciousness; 2) any disease affecting the nervous system, including dementia and psychiatric illness; and 3) a history of previous alcohol or drug abuse. The images and subsequent analyses were performed in compliance with a protocol approved by our institutional review board, and all volunteers provided informed consent.
Imaging.—From 1993 to 1997, MR images were acquired by using a 1.5-T Signa unit (GE Medical Systems, Milwaukee, WI) with version 5x software, a quadrature head coil, and a standard clinical protocol. Sagittal T1-weighted (500/11/2 [TR/TE/excitations]) images were acquired and used for localization. With the midsagittal image as a reference, coronal intermediate- and T2-weighted (3800/21, 105/2) fast spin-echo images were acquired from the genu of the corpus callosum to the splenium. Interleaved sections were acquired, with a section thickness of 3 mm. A 512 × 256 matrix was selected with a 22-cm field of view (FOV). Flow compensation, an inferior saturation pulse, and variable bandwidths were used. Axial intermediate- and T2-weighted (3000/31, 90/1) standard spin-echo images also were acquired, with a section thickness of 5 mm and an intersection gap of 2 mm. A 22-cm FOV was used with a 256 × 192 acquisition matrix. This sequence was part of our standard clinical protocol at the time. The axial images were used to calculate total intracranial volume, which was used in a correction procedure to statistically adjust for variability in head sizes.
Volumetric Image Analysis.—The coronal intermediate- and T2-weighted spin-echo images were processed by using Analyze version 6.0 (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN) on Sparc 10 workstations (Sun Microsystems, Mountain View, CA). The original 16-bit images were converted to 8-bit images in Analyze format and then permanently archived on optical disks by using a lossless compression algorithm.
Multistep volume analysis was then performed by using several image-processing tools in the Analyze program. These included multispectral tissue segmentation and region-of-interest (ROI) pixel counting. Multispectral tissue segmentation was performed in a manner similar to that described previously (2). Regions of CSF were defined by the user (C.V.A.), who traced a representative area in the lateral ventricle. Gray matter was defined by pixel signals represented in the hippocampus, and WM was defined in the temporal stem. Region samples were then plotted in a 2D feature space in which the pixel signal intensity on the T2-weighted images was on the x axis and the pixel signal intensity on the intermediate-weighted images was on the y axis. A k–nearest neighbor, or k-NN, multispectral algorithm was applied to the pixels of the entire section. When a feature space map that accurately reflected the three tissue types was obtained (with the original spin-echo images as a reference), it was applied to the remaining sections in the study. Generating separate feature space maps for the four most posterior sections was necessary because of the inhomogeneous sensitivity of the radio-frequency coil. Once the classified images accurately represented the three tissue types in all sections, they were stored and used for calculating ROI volumes.
Volumes of the following brain structures were determined by using the ROI feature of Analyze that yields a count of gray matter, WM, and CSF pixels: hippocampus, parahippocampal gyrus, fusiform gyrus, inferior temporal gyrus, middle temporal gyrus, superior temporal gyrus (Fig 1). The volumes of the temporal horn of the lateral ventricle, rhinal sulcus, inferior temporal lobe sulcus, middle temporal lobe sulcus, superior temporal lobe sulcus, and sylvian fissure were quantified as well. For gyral volumes, the total number of gray matter and WM pixels within the ROI was the basis for individual gyral volumes; CSF pixels were used to determine sulcal and temporal horn volumes. Finally, WM was measured linearly across the temporal stem by using a line connecting the inferior aspect of the sylvian fissure, where the WM border begins, to the margin where WM ends, adjacent to the temporal horn (Fig 1). This measure was outside the boundaries used to measure gyral volumes.
Each structure was manually traced, beginning anteriorly at the posterior aspect of the amygdala and continuing posteriorly to the level of the superior colliculi and medial pulvinar nucleus of the thalamus. Temporal horn volumes included the area anterior to the atria of the lateral ventricle. Volumes for each brain structure were calculated by summing the gray matter and WM pixels for each section and then multiplying the result by the voxel dimension (5.539 × 10−4 cm3). The CSF measurements were converted to volumes by multiplying them by the same voxel dimension. Linear WM measures were averaged across the sections for analyses of the right and left temporal lobes. Since the WM temporal stem measure represented a novel measure of WM integrity, we ensured careful replication of the measure by having a neuroradiologist (D.D.B.) guide the rater (C.V.A.). For all measures, rater-neuroradiologist interrater reliabilities of 0.9 or higher were achieved with a subset of images before full analysis of the entire data set was performed.
Reliability and Statistical Analysis.—By using previously described methods (2), the rater was trained under the direction of the neuroradiologist to obtain temporal lobe measurements. The average overall intrarater reliability coefficient for gyral (WM and gray matter combined) measures was 0.90, and the average reliability coefficient for CSF measures was 0.88. Quantitative measures were evaluated by using an analysis of variance. In several analyses, the t statistic was used with Bonferroni correction, because of the multiple comparisons. Significant differences were reported only after Bonferroni corrections were applied. Correlational analyses were performed by using Pearson partial correlations adjusted for age and sex. We also tested whether the quadratic function best represented the relationships by using the curve-fit analysis program of the Statistical Package for the Social Sciences (48).
Study 2: Temporal Lobe Morphology in TBI
A variety of pathologic brain changes result from TBI (61). A particular target of traumatic injury is the hippocampus, as a consequence of mechanical deformation, excitotoxic reactions, diffuse axonal injury involving hippocampal efferents or afferents, or a combination of these (43, 47, 62). We have shown that hippocampal atrophy and temporal horn dilatation are related to trauma; however, temporal horn size may not be predictive of hippocampal size (26, 47, 63). However, to our knowledge, the relationship between temporal lobe gyral and sulcal CSF volumes and their relationships to hippocampal and temporal horn volumes after TBI have not been investigated. Also, TBI may selectively damage WM more than gray matter, and CSF changes may be more reflective of WM loss than gray matter loss (31, 46, 47). As previously stated, we predicted that the position of the WM stem in the temporal lobe creates a convergence of WM pathways that are probably affected by TBI. Accordingly, in study 2 we examined patients with TBI by using the same analyses performed in study 1; the findings in study 1 provided the normative references for comparison.
Subjects.—The sample of patients with TBI consisted of 118 subjects (78 male, 40 female). All subjects met the minimum criteria for brain injury in the TBI Model Systems definition (64). Generally, they had moderate to severe brain injuries (mean Glasgow coma scale score, 8.2; SD, 3.4; range, 3–15) and were inpatients in the rehabilitation unit between 1992 and 1997. Most of these subjects participated in a previous investigation (24). The mean age of the entire sample was 21.9 years (mean age, 17–45 years). On average, imaging and neuropsychologic testing were performed more than a year after injury; in all cases, examination was performed at least 45 days after injury.
Imaging.—All imaging and image analyses were identical to those in study 1. However, in terms of subject selection, patients who underwent temporal lobectomy and those who had macerated temporal lobe(s) were excluded, because gyral and sulcal boundaries were indistinct. Nevertheless, subjects who had a history of acute temporal lobe contusions but reliably identifiable sulcal and gyral boundaries at the time of imaging were included. Accordingly, this investigation focused on the nonfocal brain injury to the temporal lobe that accompanies TBI. A neuroradiologist (D.D.B.) read all of the images.
Neuropsychologic Testing.—Eighty subjects with TBI underwent neuropsychologic testing, which included the Weschler Memory Scale–Revised (WMS-R) (63). The WMS-R can be separated into the Verbal Memory Index and the Visual Memory Index, which were used to examine select temporal lobe structures. At statistical analysis, partial correlations (in which age and sex were controlled) were determined to examine the relationships among total temporal gyral and hippocampal volumes and WM temporal stem measures in TBI.
Results
Study 1
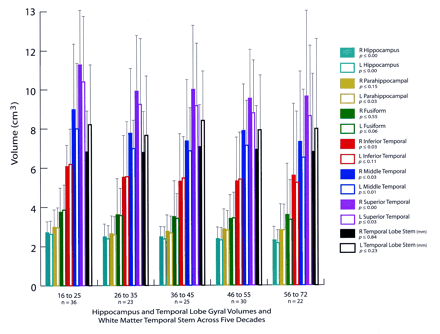
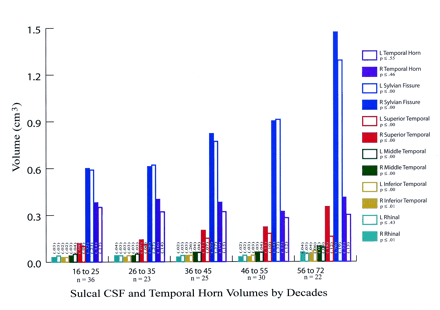
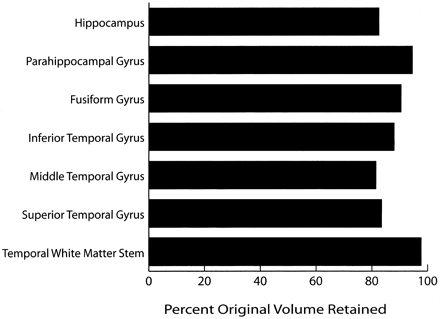
Figures 2 and 3 show the temporal lobe volumes in the healthy subjects whose ages spanned more than 5½ decades. Statistical analysis across decades revealed a significant age effect for several measures of temporal lobe gyral volume (Fig 2). The width of the temporal lobe stem, an index of WM integrity, did not significantly differ among the subjects (Figs 2 and 4). To demonstrate change over time, percentages of original gyral volumes retained are graphically displayed in Figure 4; these are based on change in the 16–25-year-old subjects compared with that of the 56–72-year-old subjects. This measure revealed that, among all measures, parahippocampal volume changed the least, with a reduction of only 5.5%. In contrast, the middle temporal gyrus had the most significant change of approximately 18.5%. All sulcal CSF volumes had highly significant age effects in which sulcal CSF volumes, except for that of the left rhinal sulcus, essentially doubled in the older subjects (Fig 3). Temporal horn CSF volume was not significantly different.
Temporal lobe structures between the two hemispheres were highly interrelated. In the comparison of homologous gyri and sulci, all right and left temporal lobe structures were significantly correlated (P ≤ .001), with an average correlation of approximately .65. Likewise, correlations between hippocampal volume and that of each temporal lobe gyrus were highly significant (P ≤ .001), with a mean correlation of .56.
The use of a correlation matrix to compare individual gyri and sulci would have been unwieldingly complex. Therefore, individual gyral and sulcal volumes were summed in both the left and right temporal lobes by decade of subject age, and the average was obtained to serve as a single total gyral or temporal lobe volume and a single total temporal lobe sulcal volume. Temporal horn volumes on the right and left sides were averaged to represent a single measure of temporal horn volume, as were the hippocampal volume and WM temporal stem measure. Total temporal gyral and sulcal volumes were only minimally correlated (r = −.16, P ≤ .05). Head size, as measured with the total intracranial volume, was significantly correlated with total temporal gyral volume (r = .65, P ≤ .001) but not sulcal volume (r = .08, P = .34). Temporal horn volume was positively correlated with both total sulcal (r = .26, P ≤ .001) and temporal gyral volumes (r = .22, P ≤ .006). Total hippocampal volume was not related to sulcal volume (r = −.05, P = .49), but it was significantly related to total temporal gyral volume (r = .74, P ≤ .001). Similarly, the total WM width in the temporal stem was correlated with total temporal gyral volume (r = .41, P ≤ .001) and hippocampal volume (r = .26, P = .001) but not total sulcal volume (r = .06, P = .468) or temporal horn volume (r = .01, P = .87).
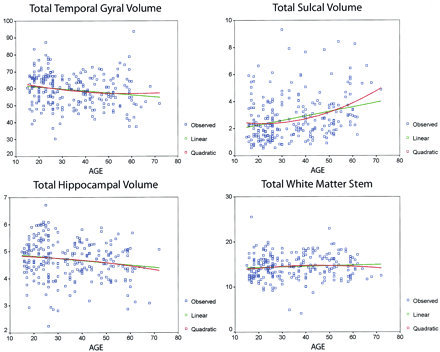
The quadratic fit was examined, but again, because of the large number of variables, this analysis was restricted to total temporal gyral volume, total hippocampal volume, total WM stem width, and total sulcal volume. When a significant difference was observed, sex differences were explored, but none were significant. Data fitted with linear as well as quadratic functions revealed that differences in total temporal gyral, sulcal, and hippocampal volumes were significant (Fig 5). Fits of the WM stem measures with linear and quadratic functions did not reveal a significant difference. Figure 5 also demonstrates the considerable variability in the temporal lobe volumes, particularly sulcal volume, in normal aging.
Study 2
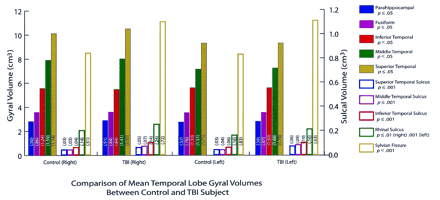
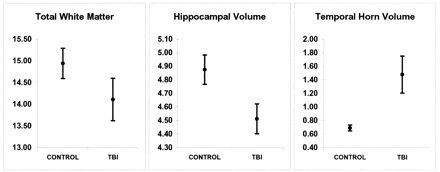
Temporal lobe morphometric results for patients with TBI are summarized in Figures 6 and 7. The distinctly different findings of hippocampal atrophy, reduced WM width, and temporal horn dilatation in patients with TBI, compared with findings in control subjects, are presented in Figure 7. Statistical analyses revealed no significant differences in any gyral volume between patients with TBI and healthy control subjects (Fig 6). In contrast, patients with TBI had significantly larger sulcal volumes in each sulcus, compared with those of control subjects (Fig 6).
Findings were consistent with the observations in the healthy control subjects. The temporal lobe gyral volumes were interrelated; these relationships included significant positive correlations between homologous gyri on the right and left sides. In patients with TBI, all correlations were significant (P ≤ .001) and ranged from .41 (right superior temporal gyrus and left fusiform gyrus) to .64 (left inferior temporal gyrus and the right inferior temporal gyrus). The same was true for the sulcal measures, with which all correlations between the right and left sides were significant (P ≤ .001). However, the range was larger than that of gyral measures; correlations varied from .26 (right sylvian fissure and left middle temporal sulcus) to .70 (left inferior temporal lobe sulcus and left middle temporal lobe sulcus). Total sulcal volume was not significantly correlated with gyral volume (r = −.13, P = .11). Also, unlike the control comparisons in which temporal horn volume was only minimally correlated with gyral volume (r = .22, P = .006) and sulcal volume (r = .26, P = .001), the correlations in patients with TBI were particularly robust for temporal horn volume and total sulcal volume (r = .40, P ≤ .001); however, temporal horn volume was not correlated with total gyral or temporal lobe volume (r = −.01). Another important difference in patients with TBI compared with control subjects was that the WM temporal stem measure, which was not correlated with sulcal volumes in control subjects, was negatively correlated with total temporal sulcal volume (r = −.30, P ≤ .001) in patients with TBI. Likewise, the WM temporal stem measure was negatively correlated with temporal horn volume (r = −.48, P ≤ .001) but not total gyral or temporal lobe volume (r = .07). Hippocampal volume was correlated with WM temporal stem width (r = .34, P ≤ .001), total sulcal volume (r = −.24, P = .004), and gyral volume (r = .67, P = .001). In TBI, the size of the hippocampus was significantly correlated with most gyral volumes but few sulcal volumes; this finding was in contrast to temporal horn volumes that were related mostly to sulcal volume but few gyral volumes (Table).
Verbal memory results, as determined with the WMS-R, correlated significantly with hippocampal volume (r = .42, P ≤ .001), total temporal gyral volume (r = .44, P ≤ .001), and temporal WM stem width (r = .25, P ≤ .03). Only hippocampal volume was significantly related to the visual memory component (r = .23, P ≤ .05).
Discussion
Study 1
In this cross-sectional study that included healthy individuals with ages spanning 5½ decades (age range, 16–72 years), aging resulted in significant but modest changes in temporal lobe parenchymal volume. When all temporal lobe gyral volumes (on both left and right sides) were combined into a single average that represented total temporal lobe gyral volume, 87% of the original gyral volume was retained, when the volume in 56–72-year-old subjects was compared with that of the 16–25-year-old subjects. This result is equal to a gyral volume loss of approximately 0.26% per year, which is consistent with the 0.3% annual change in the whole-brain volume of control subjects in the study by Fox et al (49, 50). Thus, similar to other findings from studies of aging (52–55), this volume reduction in the temporal lobe parenchyma is well less than 0.5% per year in persons aged 16–72 years. The parahippocampal, fusiform, inferior temporal gyri, and temporal stem had the smallest age-related changes, whereas the hippocampus and the middle and superior temporal gyri had the greatest changes. These observations suggest that in healthy individuals aged 16–72 years, temporal lobe parenchymal volume remains relatively stable. Likewise, temporal horn volume did not significantly increase over time.
In contrast, sulcal volumes doubled over this age range. The divergence between modest age-mediated effects on gyral volume and robust age-related changes in cortical CSF volumes in the temporal lobes requires some discussion of the differences between CSF and parenchymal measures in normal aging. Although temporal lobe CSF volume was significantly related to gyral volume, the correlation was nominal (r = .16). This finding seems somewhat counterintuitive, but it is similar to what Symonds et al (54) observed in 63 healthy individuals aged 51–82 years. They observed an insignificant correlation of −.05 between whole-brain sulcal CSF and cortical gray matter volumes. Thus, changes in sulcal CSF volume may not indicate concomitant changes in gyral volume. Similarly, Resnick et al (55), with a within-subjects prospective design, found an average increase in ventricular volume of 1.5 cm3 over 1 year (presumably due to aging), but they found no change in brain volume in 116 subjects aged 59–85 years. However, such an increase in ventricular CSF volume represents less than 0.1% of the total brain volume, and Resnick et al (57) suggest that subtle variations in parenchymal size may be reflected in CSF volume, although they are not detectable as differences in parenchymal size with current quantitative MR imaging techniques. Likely, a similar scenario is present in the current study. Another factor that detracts from the relationship between gyral and CSF volumes can be seen in Figure 5, which demonstrates that temporal lobe sulcal CSF volume did increase as temporal lobe volume decreased; however, sulcal CSF had considerably more variability. Furthermore, even doubled sulcal CSF volume represents less than 0.1% of the total temporal gyral volume. Thus, although related, gyral volume and sulcal CSF volume may be independently influenced by other factors, including age. For example, head size and gyral volume, but not sulcal volume, were significantly correlated.
Another potential explanation for these findings of disproportionate CSF and temporal lobe parenchymal changes with age exists. Bartzokis et al (56) reported both linear and quadratic changes in temporal lobe volumes in healthy men aged 19–76 years. They observed that WM volume increased until patients were aged 47 years, then declined thereafter. The methods in our study varied from those of Bartzokis et al, but differences in volumes that may reflect maturational changes early in adulthood and age-related degeneration later in life might account for some of the variations. Figure 5 clearly demonstrates significant differences in the slopes of the curves. Also, although the quadratic fit of the WM stem data did not indicate a significant difference (Fig 5), the current findings support those of Bartzokis et al, in which the peak of WM values occurred in patients aged 40–50 years.
Clinically, the size of the temporal horn has been a common reference point in discussions of the integrity of the hippocampus. However, in this control sample, temporal horn and hippocampal volumes were not significantly related. As mentioned, hippocampal volume was significantly related to head size and total gyral volume but not sulcal volume. Regarding individual gyri, the hippocampal volume was positively and significantly correlated with those of all temporal lobe gyri, with no clear predominance of any gyrus, although principal hippocampal input is provided by the parahippocampal and fusiform gyri. We explored this finding even further with multiple regression analyses, but all models with significant results included most, rather than select, temporal lobe gyri, with no unique contribution from the parahippocampal or fusiform gyri. Hippocampal volume was significantly correlated with the temporal stem WM measurement, but the magnitude of this correlation was about half (or only 6% of explained variance) of that observed with gyral volume (or about 30% of explained variance). The robustness of the gyral volume correlations with the hippocampal volume, combined with the lack of correlations between the hippocampus and temporal horn or other CSF measures, further underscored the relative independence of hippocampal size and the size of brain regions that contain CSF (in particular, the temporal horn that forms a border with the hippocampus).
In contrast to the modest changes in gyral volumes in the subjects whose ages spanned 5½ decades, the sulcal volumes (except for that of the left rhinal sulcus) significantly increased in all cases. In light of the minimal changes in actual gyral volumes, this observation is interesting. As argued previously, changes in temporal lobe CSF volume with normal aging were not specific to brain parenchymal changes or related to head size. In pathologic conditions (study 2), changes in CSF morphology often are thought to reflect structural changes. However, as demonstrated in this study, the relationship in normal aging is far from linear, and the change in CSF volume associated with aging is not a simple reflection of the loss of parenchymal volume.
While differences between left and right gyral and sulcal volumes were present, the correlations between the left and right sides were generally high, and all were significant. Nonetheless, even the highest correlation (r = .86, CSF volumes in left and right sylvian fissures) accounted for only 74% of the explained variance. Accordingly, although they were highly interrelated, the left and right structures differ in size, and the homologous gyri (or sulci) are not exact duplicates. Although the data are not presented in tabular form (because of the enormity of the table), all gyri were compared with all other temporal lobe gyri; positive and very significant correlations were observed across all temporal lobe gyral structures, regardless of hemisphere. The same result was true for similar comparisons of temporal lobe sulci. These findings suggest that, in healthy subjects aged 16–72 years, morphologic changes tend to be bilaterally uniform across all gyri and sulci. A clinical caveat to this symmetry exists. Since a particular gyrus (or sulcus) typically is similar to its contralateral counterpart, deviations in a temporal lobe gyrus from its homologue may indicate a pathologic condition in the gyrus. With regard to symmetry of the hippocampus, as with gyral and sulcal morphometry, the right and left sides were highly interrelated. However, Jack et al (13–15), Geroldi et al (57), Utsunomiya et al (17), Gunten et al (58), and we (24) demonstrated that the right hippocampal formation is slightly larger than its left counterpart. This difference also was observed in this study and maintained in the subjects aged 16–72 years. The rate of reduction in hippocampal volume averaged less than 0.25% annually.
In this study, we did not have a sufficient sample size to investigate sex differences by decade of patient age. Also, we did not analyze the amygdala, fornix, mammillary bodies, or cingulate gyri, important limbic structures that should be examined in relationship to the temporal lobe structures discussed herein.
General Discussion
In this sample of patients with TBI, significant hippocampal atrophy and WM stem width reduction occurred, along with temporal horn dilatation, but specific temporal lobe gyral volume loss was not observed. Before these findings are discussed further, it should recalled that the focus of this investigation was to examine the nonfocal, nonspecific changes in temporal lobe structures that accompany TBI. Accordingly, patients with TBI and focal or major encephalomalacic changes that obscured temporal lobe boundaries were excluded. Even so, surprisingly, no significant reduction in temporal lobe gyral volume was observed in this sample of patients with TBI. Since prominent bilateral changes in sulcal CSF volumes were present in connection with WM and hippocampal atrophy, the nonfocal changes in temporal lobe morphology after TBI appear to occur not in the gyri but in the hippocampus and subcortical WM. Since, among other structures, the axon is most susceptible to the shear, strain, and tensile effects of injury (64), the findings from study 2 appear to be most consistent with pathologic injury related more to WM damage than to gray matter damage (46). In such a scenario, WM pathways may have damage that results in WM atrophy, cell body preservation, and hence, the lack of change in overall temporal lobe gyral volume. Following this logic, the fact that gyral volume changes were not observed may not be surprising. Cell bodies may survive injury to the axon, with the neuron rendered dysfunctional (65). Ultrastructural studies of DAI have revealed normal-appearing axons adjacent to injured ones and apparently intact perikarya (66, 67). If axotomy does not occur, the damaged axon may still become physiologically dysfunctional, but neuronal death does not ensue; therefore, the cell body is preserved (68). Recently, MR spectroscopic studies have revealed abnormal WM findings on clinically normal images (46). With the method of temporal lobe volume calculation in the current study, gray matter contributed substantially to each gyral volume. Reduction in the WM temporal stem width was a direct gauge of WM integrity in the temporal lobe of patients with traumatic injury. Thus, the lack of substantially reduced gyral volume and notable reduction in temporal stem WM may reflect the preservation of cell bodies in the cortical mantel of the temporal lobe after injury but not the disruption of subcortical WM pathways. Also, our methods for determining parenchymal volume may have been insensitive to the microscopic pathologic process that occurs in TBI (27).
Pathologic differences in WM and gray matter occur in aging (69) and in a variety of disorders, including Alzheimer disease (51, 70), schizophrenia (18), radiation necrosis (55), and multiple sclerosis (44). The data from this investigation indicate that, in patients with TBI but not focal temporal lobe injury, the presence of DAI likely appears as bilaterally reduced WM at the level of the temporal stem. Temporal horn enlargement also was bilateral, as was hippocampal atrophy.
Several other observations were consistent with the reasoning that TBI results in selective injury to WM that appears as cortical CSF changes rather than specific gyral volume losses. In the injured brain, total sulcal CSF volume in the temporal lobe was not significantly correlated with total gyral volume. Despite substantial increases in sulcal CSF volume, this lack of a significant relationship with gyral volume suggests that independent processes influence the increase in sulcal CSF volume in patients with TBI. As postulated, if WM volume is reduced, overall brain volume decreases, and sulcal and ventricular CSF passively increases to fill the void (2). Because ventricular CSF provides an outward pressure gradient, it may assist in maintaining the overall shape and configuration of the temporal lobe after injury. If this is the case, temporal horn expansion in response to surrounding subcortical WM loss at the level of the temporal stem does not necessary correlate with gyral volume; this was our observation (temporal horn correlation with gyral volume, r = −.01). Furthermore, it would be expected that, in TBI, a significant negative correlation between temporal horn volume and WM volume exists; it did (temporal horn volume correlation with the WM stem measurement, r = −.48, P ≤ .001). To explore these relationships further, we performed a number of multiple regression analyses. Regardless of the model, only the WM measure contributed to temporal horn volume. Accordingly, increased temporal horn volume is associated with a decrease in the amount of WM, and decreased WM volume likely is the basis for temporal horn dilatation in TBI. WM surrounds the temporal horn, with the exception of the boundary of the hippocampus and amygdala. Thus, temporal horn dilatation in TBI is more an indirect index of the integrity of temporal lobe WM than an indirect index of hippocampal atrophy. On the basis of this reasoning, we speculate that temporal horn dilatation in TBI predominantly reflects WM changes at the temporal lobe level, whereas hippocampal atrophy occurs as a consequence of direct injury, such as local trauma–induced excitotoxicity or transneuronal degeneration affecting hippocampal cell body integrity (45, 60, 71, 74).
Intuitively, CSF volume has been assumed to change in concert with brain parenchymal volume. The current findings suggest that, in TBI (as well as with aging), the relationship between CSF and brain parenchyma is multifactorial, with no simple linear relationship. As previously discussed, Symonds et al (56) examined sulcal CSF volume in relation to WM and gray matter volumes in a variety of neurologic disorders, although TBI was not included. They found that sulcal CSF volume was more predictive of WM than of gray matter changes. Reddick et al (75), in a postirradiation study in children with brain neoplasm, found WM volume losses, whereas gray matter volumes remained relatively unchanged. Consistent with Reddick et al, we also argue that sulcal CSF and temporal horn volumes are related to WM atrophy in TBI, whereas gyral volume measures are insensitive to these trauma-induced changes.
Obviously, considerable pathophysiologic conditions may exist in structures that may appear anatomically normal (46, 68). In the current TBI study, distinct WM and hippocampal atrophic changes were observed. The fact that gyral volumes did not change should not be interpreted as an implicit indication that normal cellular function was present. In fact, the neuropsychologic findings demonstrated that temporal lobe and hippocampal volume, along with the width of the temporal stem, were related to memory outcome. Basically, a reduction in size in any of these structures was associated with reduced memory performance, as measure with the WMS-R.
One final clinical note should be mentioned. Since aging had only a minimal effect on temporal lobe morphology in study 1 (at least in subjects aged 16–72 years), age appears to be an unlikely contributor to trauma-induced changes in the temporal lobe that occur in patients with head injuries, as revealed in study 2. Confirmation of this finding was observed at statistical analysis, since age, as a covariate, had no influence.
Conclusion
In the healthy brain, temporal lobe morphology remained relatively stable in patients whose ages ranged more than 5½ decades (ie, in subjects aged 16–72 years); gyral volume decreased by approximately 0.26% per year. In contrast, sulcal CSF volume essentially doubles. During this period, hippocampal volume decreases minimally yet significantly, and temporal horn volume increases, signifying age-related changes. Gyral volume is related to hippocampal volume, and both are related to head size. Although most hippocampal input occurs via the parahippocampal and fusiform gyri, no unique relationship between overall volume of these gyri and hippocampal volume was present. Likewise, in the healthy brain, temporal lobe volume and the width of the WM temporal stem were relatively independent. Temporal horn volume is relatively unrelated to hippocampal volume.
Trauma changes the pattern of morphometric relationships among temporal lobe structures. Substantial increases in sulcal CSF volume appear to be related to WM atrophy and not to specific changes in gyral volume. Although hippocampal atrophy was prominent, temporal horn dilatation was related more to WM atrophy than to hippocampal atrophy. Changes in sulcal CSF volume were relatively independent of gyral volumes. Thus, both subarachnoid and ventricular CSF volumes appear to be more sensitive to WM atrophy than to changes in gyral volume in patients with TBI. The reduced size of temporal lobe structures in TBI was significantly associated with poorer memory performance.
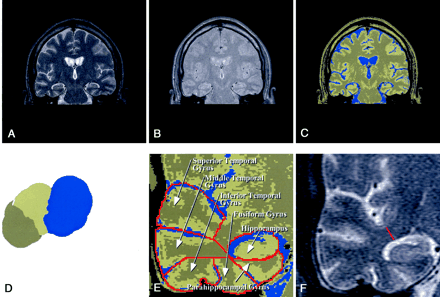
Images used in volume determination.
A, Coronal T2-weighted MR image.
B, Coronal intermediate-weighted MR image.
C, Segmentation image from A and B.
D, Feature space showing separation of CSF (blue), white matter (khaki), and gray matter (tan).
E, Close-up segmented image of the right temporal lobe depicts the hippocampus and five temporal gyri.
F, The red line indicates the length of WM from the base of the superior temporal sulcus to the base of rhinal sulcus and defines the temporal stem measurement. The linear distance (in centimeters) provided the basis for this measure, summed across all sections.
Bar graph shows hippocampal and temporal lobe gyral volumes, along with WM temporal stem measurements grouped by subjects’ ages (in years). The P values are based on analysis of variance comparisons across the decades in which significant changes may have occurred. Note the significant age effects on hippocampal volume and several gyral volumes, although considerable variability exists, as represented by the SD bars. All measures are in cubic centimeters3, with the exception of the temporal stem linear measure, which is in millimeters.
Bar graph shows sulcal CSF and temporal horn volumes grouped by subjects’ ages (in years). The P values indicate whether a significant change in volume by decade was present. The number on the bars are the SDs. Note the consistent and highly significant increases in CSF volumes (except for that of the left rhinal sulcus) with aging.
Bar graph shows the percentage of original volume retained in each temporal lobe structure, as determined by comparing the value in 16–25-year-old subjects with that in 56–72-year old subjects. Most structures, particularly the temporal WM stem, retain a large percentage of their original volume over time.
Scatterplots show total temporal gyral and sulcal volumes, hippocampal volumes, and temporal stem measurements, fitted with linear and quadratic functions. Note the greater variability in sulcal volume compared with the parenchymal measures. At statistical analysis, degrees of freedom for regression and residuals, respectively, were 2 and 251 for the linear function and 1 and 252 for the quadratic function. For each structure, values with the functions were as follows: total gyral volume, quadratic F = 4.35 and P ≤ .014, linear F = 7.97 and P ≤ .005; total sulcal volume, quadratic F = 14.09 and P ≤ .00001, linear F = 24.06 and P ≤ .00001; total hippocampal volume, quadratic F = 3.65 and P ≤ .03, linear F = 7.14 and P ≤ .008; and total WM, quadratic F = 1.2 and P ≤ .29, linear F = 1.41 and P ≤ .24. In each case, head size (total intracranial volume) and sex were used as covariates.
Bar graph shows the mean for volumes in the comparison of healthy control subjects and patients with TBI. In each case, TBI resulted in significant atrophy (P ≤ .01). The number on the bars are the SDs.
Graphs show the total WM measure in the temporal stem, hippocampal volume, and temporal horn volume. In each case, TBI resulted in significant atrophy (P ≤ .01). The bars indicate the SDs.
Pearson correlation values in patients with TBI
Acknowledgments
The technical assistance of Tracy Abildskov and statistical assistance of Kris Kristensen, PhD, are gratefully acknowledged, as is the editorial assistance of Jo Ann Petrie.
Footnotes
Partial funding for this study was provided by the Ira Fulton Foundation.
References
- Received November 9, 2000.
- Accepted after revision August 17, 2001.
- Copyright © American Society of Neuroradiology