Abstract
BACKGROUND AND PURPOSE: Laboratory studies have been used to identify nitric oxide as a notable mediator in neuronal death after acute brain injury. To our knowledge, this has not previously been confirmed with in vivo study in humans. Our purpose was to seek in vivo evidence for the induction of nitric oxide synthase (NOS) in human acute brain injury by using proton MR spectroscopy.
METHODS: In vitro proton MR spectra were obtained in neural extracts from 30 human cadavers, and in vivo spectra were obtained in 20 patients with acute brain injury and in a similar number of control subjects.
RESULTS: We identified a unique peak at 3.15 ppm by using in vivo proton MR spectroscopy in eight of 20 patients with acute brain injury but not in 20 healthy volunteers (P < .002). On the basis of in vitro data, we have tentatively assigned this peak to citrulline, a NOS by-product.
CONCLUSION: To our knowledge, our findings suggest, for the first time, that excitotoxicity may occur in human acute brain injury. Confirmation with the acquisition of spectra in very early acute cerebral injury would provide a rationale for the use of neuroprotective agents in these conditions, as well as a new noninvasive method for quantification.
The pathogenesis of neuronal death after injury to the brain, from any cause, has been the subject of considerable investigation. Numerous causative mechanisms have been identified in animal models; these include early and delayed ischemia, direct neuronal damage, and mobilization of secondary metabolic pathways with the production of inflammatory mediators (1). Many of these are linked to the excitatory hypothesis, in which brain injury from a variety of causes stimulates the release of large quantities of excitatory neurotransmitters—especially glutamate—that result in an increase in intracellular calcium levels (2). This effect is believed to activate nitric oxide synthase (NOS), with the production of free nitric oxide (NO), which subsequently causes DNA denaturation, energy depletion, and cell death (1, 2). To our knowledge, this has not been previously confirmed in humans.
Over a 5-year period of involvement in human in vivo proton MR spectroscopy (a technique capable of noninvasively providing metabolic information about the brain in a range of conditions, including stroke, infection, and neoplasms), we had examined numerous patients with acute brain injury, as well as those with metabolic and neoplastic conditions, and routinely measured relative levels of the well-described metabolites: N-acetylaspartate, creatine, choline, lactate, and glutamate (3, 4). Occasionally, in some patients with acute brain injury (including acute cerebral infarction, head injury, perinatal asphyxia, and cerebral radionecrosis), we identified an additional small spectral peak halfway between the creatine and choline peaks. We noted these incidentally, but we were unable to categorize them, because we were not able to find descriptions of this peak in the literature. In an attempt to identify this unknown peak and to determine its importance we conducted two separate investigations: 1) an in vitro proton MR spectroscopic study of human brain and spinal cord neural extracts and 2) a prospective examination of patients referred for in vivo proton MR spectroscopy after acute brain injury. We performed these in combination with examinations in a control group of healthy volunteers.
Methods
Neural Extract Studies
Specimens of parietal cortex (n = 7), cerebellum (n = 5), cervical spinal cord (n = 15), and thoracolumbar spinal cord (n = 3) were obtained from 30 human cadavers within 48 hours of death. These were frozen at −60°C, cut into thin strips, lyophilized, and placed into 5-mm borosilicate tubes filled with D2O and a known quantity of 3-trimethylsilylpropionate. With all samples, proton MR spectroscopy was performed with a Bruker AMX 300 machine operating at 7 T by using previously described techniques (4). The one-dimensional spectra were obtained at 303K by using a 30° pulse width, 6-second repetition rate, 3030-Hz sweep, 16k digital resolution, and total acquisition over 2.7 seconds. Each spectrum was the sum of 1024 free-induction decays. The signal was processed by using line broadening of −1 Hz and 0.05 gaussian multiplication. Additional two-dimensional total correlation spectroscopic (TOCSY) spectra were obtained in three samples (two brain, one cervical cord), which were analyzed at spectroscopy by using a 600-MHz Bruker spectrometer at 14 T, to assist with peak assignment. No major differences in the location of peaks were identified on visual comparison of extract spectra from the four specimen groups.
Live Human Studies
The institutional ethics committee approval was granted prior to study commencement, and all subjects (or guardians) provided informed consent. Conventional MR images and single-voxel spectra were acquired with a 1.5-T system (Vision; Siemens, Erlangen, Germany). In the 18 healthy volunteers (age range, 16–57 years; mean, 34.5 years) and two healthy neonates, an 8-cm3 voxel was selected in the high parietal region, to include both cortical grey and white matter. In the 20 patients (age range, 0–76 years; mean, 33.0 years) with acute brain injury, which included acute cerebral infarction (n = 5), head injury (n = 5), perinatal asphyxia (n = 3), and cerebral radionecrosis (n = 7), examinations were conducted within 48 hours of their clinical presentation. An 8-cm3 voxel was selected within the area of abnormal brain identified at conventional MR imaging, and spectroscopy was performed by using previously described techniques (3). Water suppressed proton MR spectra were obtained with a 90°-90°-180° pulse sequence with a 2-second interpulse delay. We used a spin-echo refocussing time (TE) of 20 milliseconds, TR of 1500 milliseconds, 240 × 240-mm field of view, and 16 × 16 phase-encoding steps with one NEX per step. Each water-suppressed spectrum was divided by a non–water-suppressed spectrum obtained immediately before, by using similar acquisition parameters, with optimized shimming for B0 homogeneity within the selected voxel.
Data were postprocessed by using NUMARIS software (Siemens), including zero-filling to 32k, 1-Hz exponential multiplication, Fourier transformation, zero-order phase correction, and baseline correction. Spectral peaks were assigned on the basis of previously published data, and integrated by using prior knowledge integral shapes (3, 4). Total imaging and spectral acquisition time averaged 53 minutes across both groups of subjects. The presence of citrulline was defined as the clear visualization of a discrete peak, with upward convexity, between the creatine (3.04-ppm) and choline (3.21-ppm) peaks.
Final diagnoses in the patients with acute brain injury were established by using either histologic examination (patients with radionecrosis) or 6-month clinical follow-up. The statistical significance of the difference between the two groups in the observation of citrulline was calculated by using the Fisher exact test (5). Given that the overall total of subjects numbered 40, the upper limit for this test and confirmation with a χ2 test (Yates corrected), was sought (5); both yielded an identical result.
Results
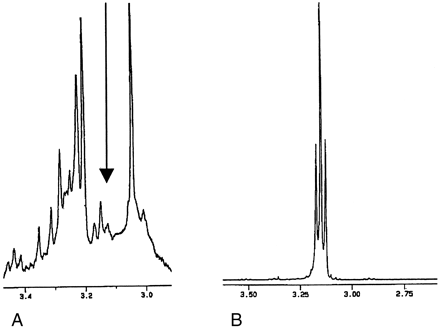
In the in vitro spectra, in the region between the creatine (3.04 ppm) and choline group of peaks (from 3.21 ppm) we identified a small triplet in every specimen that was centred at 3.15 ppm (Fig 1A). Given that the characteristics of this peak suggested that it corresponded to a −CH group, we subsequently obtained spectra from a range of amino acids, organic acids, and carbohydrates. The triplet corresponded most closely to the δCH2 of citrulline (Fig 1B), and this was confirmed with a spiking experiment.
In vitro identification of an unknown peak.
A, Note the small triplet (arrow) between the creatine and choline peaks in this magnified section of an MR spectrum obtained in a cerebral cortex specimen.
B, Magnified region of this citrulline spectrum corresponds to the shape and location of the unknown peak.
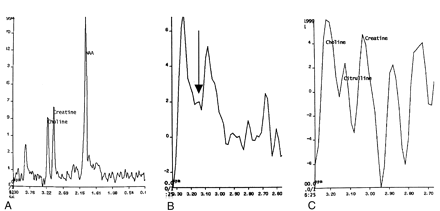
In the in vitro spectra, we could not identify any peaks between creatine and choline in the 20 healthy volunteers (Fig 2A). In contrast, eight patients in the brain injury group (two with cerebral infarction, two with head injury, one with perinatal asphyxia, three with radionecrosis) had a visible peak between creatine and choline centered at 3.15 ppm, which we believe represents citrulline (Fig 2B and C). The observed difference between the healthy volunteers and the patients with brain injury in identification of citrulline was highly significant (P < .002) with both the Fisher exact test and the χ2 test (Yates corrected). We also calculated metabolite ratios for citrulline against creatine, with values ranging from 0.023 to 0.35. No calculation of absolute values of citrulline was possible, because relaxation parameters for this compound are unknown at 1.5 T (4).
In vivo identification of an unknown peak.
A, In vivo spectrum obtained in a healthy volunteer demonstrates the absence of any visible peak between those of creatine and choline.
B, Spectrum from an area of histologically proven radionecrosis in a 22-year-old man demonstrates a small peak centered at 3.15 ppm (arrow).
C, Spectrum from an area of suspected infarction (confirmed at 6-month clinical follow-up) in a 58-year-old woman demonstrates a large citrulline peak between those of creatine and choline.
Discussion
The in vivo identification of citrulline in human brain injury has major implications for the understanding of the pathogenesis of cerebral injury. The brain lacks ornithine, citrulline transcarbomylase, and carbamyl phosphate synthetase I, and is, therefore, unable to synthesize citrulline via the urea cycle (6). Instead, the only known method for cerebral production of citrulline is via conversion from arginine through NOS (2). Thus, definite demonstration of citrulline within brain tissue would be indicative of the induction of NOS, with concomitant production of NO. Our identification of substantial levels of citrulline in postmortem neural extracts must reflect the profound hypoxia that occurs at the time of death and the subsequent massive glutamate release that results in increased intracellular calcium levels and the induction of NOS. While this, in itself, is of some interest, it merely confirms the results that others (1, 2) have obtained in living brain preparations and small animal experiments by others. Far more exciting is our demonstration of a visible peak at the corresponding chemical shift in the in vivo spectrum in human brain injury; we have tentatively assigned this to citrulline. Confirmation that this represents citrulline would prove for the first time (to our knowledge) that excitotoxicity occurs in this set of conditions. If this method were indeed an in vivo technique for the detection of NOS catalytic activity, one would expect a more notable elevation of citrulline levels in the very early stages of acute brain injury (less than 3 hours) after the insult, and investigations of this nature are required to confirm our conclusions.
Are sufficient amounts of citrulline present for in vivo MRS measurement 48 hours after injury? Or, conversely, could the 3.15-ppm peak represent merely noise? Although, to our knowledge, no reports of the absolute concentrations of citrulline after brain injury have been published, evidence of a biphasic induction of NOS certainly exists. After injury, NO production has an early peak, which lasts less than 1 hour, and a delayed increase in NOS activity occurs; this reaches its peak at 24 hours and lasts for several days (7). If the 3.15-ppm peak represents noise, ours would probably have been the only group to observe it. We reviewed previously published in vivo spectra in patients with acute cerebral injury and, in one of the earliest descriptions of MR spectroscopy in acute stroke (in 1992), we identified an unlabeled peak at 3.15 ppm in a 5-day-old cerebral infarct that was not visible in a control spectrum on the contralateral side (8). A similar in vitro peak was also visible in one of the earliest published studies of MR spectroscopy in rabbit cerebral autolysis (in 1988), in which a 3.15-ppm peak was not present in an extract obtained 30 minutes after death, but it was clearly visible (although not labelled) at 24 hours after death (4).
Our failure to visualize the proposed citrulline peak in some patients could be explained by the relatively low sensitivity of in vivo MR spectroscopy, but the variable response to selective glutamate N-methyl-d-aspartate (NMDA) receptor antagonists and NOS inhibitors in patients with acute brain injury may also be due to nonuniform induction of NOS (1). Early quantification of NOS activity may be proven useful in the selection of patients who are likely to respond to these agents (9). In addition, we now have a notable rationale for the routine examination of patients with acute brain injury with MRS, given that information about the pathogenesis of neuronal death may be obtained directly and noninvasively.
Conclusion
The definite identification of excitotoxicity in human acute brain injury would confirm the experimental data in animal models and provide a rationale for the use of neuroprotective agents in these conditions. We present in vivo proton spectroscopic data, based on in vitro spectra on human neural extracts, that suggest that a by-product of excitotoxicity may be present after acute brain injury. Confirmation of our findings with the acquisition of serial spectra in very early acute brain injury will have profound implications on the understanding and treatment of acute brain injury in humans.
Footnotes
The work was funded, in part, by grants from the RANZCR Research Fund and the Canberra Hospital Private Practice Fund.
- Received May 9, 2001.
- Accepted after revision August 17, 2001.
- Copyright © American Society of Neuroradiology