Abstract
BACKGROUND AND PURPOSE: Transarterial embolization of cerebral arteriovenous malformations (AVMs) has been associated with postprocedural neurologic complications in 7–39% of patients. We sought to determine whether a method of targeted neurologic and cognitive testing during AVM embolization reduces the incidence of focal cognitive and other neurologic deficits associated with the procedure.
METHODS: A cognitive neurologist extensively examined 12 patients prior to AVM embolization. In each patient, a battery of tests tailored to their specific abilities was developed by using stimuli selected from standard and experimental cognitive tests to probe specific brain regions related to the location of the AVM. In each feeder vessel to be embolized, a 50-mg bolus of sodium amobarbital was superselectively administered through a microcatheter; this was followed immediately by neurologic and cognitive testing with the tailored battery. After testing, the position of the microcatheter tip was checked with fluoroscopy. If the provocative test results were negative, the evaluated feeder was embolized with N-butyl cyanoacrylate glue.
RESULTS: Although results with 27 of 29 provocative amobarbital injections were negative, results with two injections in two different individuals revealed cognitive deficits during tailored provocative testing. In both, the evoked deficits resolved with dissipation of the amobarbital effect; the feeder vessels were not embolized. Neurologic and cognitive evaluation after each of 27 embolizations revealed no major or minor deficits.
CONCLUSION: In our experience, provocative amobarbital testing prior to AVM embolization was helpful in identifying vascular territories where embolization may lead to neurologic and cognitive deficits.
Selective amobarbital tests have been shown to be effective in determining anterior versus posterior language (1) and verbal memory localization (2) in the presurgical examination of epilepsy patients. Clinical neurologic evaluation with superselective amobarbital testing prior to cerebral arteriovenous malformation (AVM) embolization has largely been ignored or limited to gross tests of strength, sensation, and visual fields (3); more recently, motor evoked potentials have been used to assess the motor pathways alone (4). In addition to vessel perforation and postembolization hemorrhage, the potential risks of AVM embolization include focal neurologic deficits related to incidental embolization of vessels that feed the eloquent cortex. Such procedures could lead to deficits in sensorimotor and higher cognitive functions. Previous reports (5) of treatment outcomes after AVM embolization have classified results such as monoparesis and hemisensory loss as “minor neurologic deficits,” and often, cognitive functions are not assessed. Reported transient and permanent complication rates have varied from 7% to 39% (6–9); these were attributed to a combination of procedure-related hemorrhage, ischemic events, and untoward embolization of vessels feeding the eloquent parenchyma.
We sought to minimize one such risk by identifying the brain functions associated with each feeder vessel by using a focused, tailored, superselective amobarbital injection prior to embolization. We describe a safe and effective method of neurologic and cognitive testing with amobarbital injection prior to AVM embolization that is tailored to each patient’s preprocedural abilities and the location of the vessels to be embolized.
Methods
Patients
A total of 14 consecutive patients with cerebral AVMs were examined for tailored neurologic and neuropsychologic testing during embolization procedures, in which 12 underwent glue embolization. The other two were directly referred for radiosurgery on the basis of findings from repeat angiographic evaluation. Of this group of 12, eight were female and four were male, with an average age of 37.2 years (SD, 10.4). In total, 29 feeder vessels were injected with amobarbital, with an average of 2.4 injections per patient and an average of 41 days (SD, 16) between embolization sessions in each patient. Among the 12 patients, AVM locations were distributed as follows: three frontal (two left, one right), one parietal (left), one frontoparietal (left), two parieto-occipital (right), two occipital (one left, one right), two temporal (left), and one cerebellar. Eight of the 12 underwent postembolization radiation therapy (at the time of this analysis). The nature of the embolization procedure and the role of neurologic and/or cognitive evaluation during the procedure were fully explained to the patients, and informed consent obtained from each.
Angiographic Technique
All procedures were performed via a standard femoral arterial approach by using a 6F introducer sheath, with patients receiving local anesthesia. An initial bolus of 5000 units of heparin was administered immediately after the arterial access was obtained, and a preliminary architectural and hemodynamic evaluation of the AVM performed by using biplane digital subtraction angiography (DSA). A 6F guiding catheter (Envoy; Cordis) was then positioned in the principal supplying arterial trunk, that is, in the cervical portion of an internal carotid or vertebral artery, and a flow-guided microcatheter (Elite or Spinnaker; Boston Scientific) was advanced toward the AVM nidus with fluoroscopic and roadmap control. Once the tip of the microcatheter reached an adequate position within or close to the AVM nidus, a superselective angiogram was obtained. Besides providing critical information about the portion of the nidus vascularized by the selected feeder and the potential presence of branches supplying healthy cerebral tissue, this superselective angiogram delineated the arterial territory to be evaluated with provocative testing. At this point, the angiography table was moved to provide the cognitive neurologist (L.R.M.) with access to the patient for testing. A manually injected 50-mg bolus sodium amobarbital in 1 mL of normal saline was superselectively administered through the microcatheter; neurologic and cognitive testing immediately followed. After completion of the testing, the position of the microcatheter tip was checked with fluoroscopy. If the provocative test results were negative, the evaluated feeder was embolized with N-butyl cyanoacrylate (NBCA) glue (Histoacryl; Braun Surgical, Meisunaen, Germany). The total time from final microcatheter positioning to the conclusion of provocative amobarbital testing and decision-making regarding the safety of embolization was 2–4 minutes.
Cognitive Testing Technique
Patients with intracranial AVMs who were scheduled for endovascular embolization (n = 14) underwent preembolization neurologic and cognitive examinations with the neurologist, who had subspecialty training in the assessment of deficits in language, memory, attention, arithmetic, and visual-spatial functions to establish their performance. On the basis of the patient’s specific performance with this extensive cognitive testing, the cognitive neurologist’s interpretation of the data, and the specific site and size of the AVM demonstrated on MR images and/or cerebral angiograms, brief (<4-minute), customized batteries of tests and stimuli (unique to each patient) were selected for presentation during the embolization session. Multiple versions of each test battery, each of equivalent difficulty and at a level appropriate for the patient, were prepared for use both during provocative testing and after each embolization. Tasks and stimuli were selected and modified from standard and experimental cognitive tests designed to probe specific brain regions so that the tasks could be quickly completed with the patient lying still on the angiography table. In the angiography suite, provocative testing with 50 mg of sodium amobarbital in each feeder vessel to be considered for embolization was performed to determine whether temporary neurologic or cognitive deficits resulted. Performance that was less than that patient’s previously established baseline level was considered a deficit. If a deficit was detected, that vessel was not embolized. If no deficit was detected, the feeder vessel was embolized, and, within 15 minutes of embolization, another round of neurologic and cognitive testing was performed to assess potential postembolization deficits.
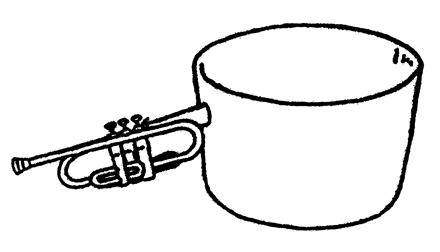
Examples of the neurologic and cognitive tests used are shown in the Table (an example of a test object is shown in Fig 1). AVMs spanning multiple lobes were tested with appropriate combinations of the stimuli described. Although language is generally thought of as being left-hemisphere dominant, and visual-spatial processing is commonly considered a predominantly right-parietal function, ample evidence suggesting that many such functions can be bilateral led us to choose the same tasks for left- or right-hemisphere AVMs on the basis of the lobe and the individual’s baseline performance.
An example of a non-real object: In this case, a pot with the outline of a trumpet is where the handle is expected.
Representative cognitive and neurologic functions assessed during provocative amobarbital testing*
Results
Of the 29 tailored provocative neurologic and cognitive amobarbital tests, 27 yielded no identifiable deficits. NBCA glue embolization was performed in the 27 vessels thus identified, and none of the patients had major or minor, temporary or permanent embolization-related sequelae at postembolization testing performed within 15 minutes of the glue procedure. No hemorrhages occurred in the 12 patients during or after any of the embolization sessions. Two superselective provocative amobarbital injections induced focal cognitive deficits that were neuroanatomically attributable to the eloquent cortex in the region of the AVM. In each patient, the deficits resolved within 4 minutes of the injection; this finding was consistent with the abatement of the drug effect. Glue embolization was not performed in the two vessels thus identified, and both patients did not have postangiographic deficits. Details of these two subjects are discussed next.
Case Illustration 1
This 27-year-old right-handed woman first presented at age 16 with episodes of speech difficulties, right hemiparesis, and right hemisensory loss lasting several hours. Phenytoin administration was started for seizure prophylaxis. CT scanning of the head followed by angiography lead to the identification of a 5–6-cm left perirolandic AVM. (Fig 2A). She was initially followed up medically and advised to avoid participating in contact sports. At age 25 years, she had a baby by means of cesarean delivery without incident. Despite anticonvulsant therapy, the number of episodes of right hemisensory loss and speech difficulty increased to four or five per month; this prompted her to seek more definitive therapy. The size and location of the AVM precluded surgical resection without the possibility of a major neurologic deficit. Her neurosurgeon referred her to our interventional neuroradiology group for endovascular embolization prior to possible radiation therapy.
DSA images in a 27-year-old woman with a left supratentorial AVM.
A, Initial diagnostic angiogram of the left common carotid artery, lateral view, shows a large AVM nidus in the left perirolandic region. Multiple arterial feeders arise from the superior and inferior division of the left MCA. Note the early opacification of the three dilated veins draining into the superior sagittal sinus.
B, During the third embolization session, the tip of the flow-directed microcatheter is advanced into a feeder that topographically corresponds to the anterior parietal branch of the left MCA. Superselective angiogram obtained prior to possible embolization shows opacification of a discrete portion of the nidus and an enlarged parietal vein, but it shows no evidence of normal arterial structures or parenchymal blushing. The amobarbital test is performed with the microcatheter tip in this position.
C, After the documentation of cognitive deficits that correlate with the left parietal cortex during the amobarbital test, the microcatheter is repositioned to a slightly more distal location, and a another superselective angiographic study is performed. This second angiogram clearly reveals the presence of two previously undetected arterial branches with a normal appearance that arise from the large feeder; these are responsible for parenchymal blushing.
The patient was scheduled to undergo staged embolization of the AVM with provocative amobarbital testing, as described before. Prior to her first embolization session, she was examined by means of cognitive neurology testing, which included an extensive cognitive test battery (3 hours). She had no notable cognitive deficits with this baseline testing. The specific tasks and stimuli to be presented during her angiographic procedures were chosen on the basis of the frontoparietal location of her AVM (Table). During her first embolization session, she underwent provocative amobarbital testing with the injection of two different left middle cerebral artery (MCA) feeding vessels. During each injection, the strength and sensation in her right arm and her verbal fluency, language, calculation, and visual-perceptual skills were assessed; all were found to be intact. On the basis of the lack of deficits with these provocative amobarbital injections, each vessel was embolized, with no postembolization deficits. A second embolization session was successfully undertaken 6 weeks after the first; during this session, provocative amobarbital testing and embolization of a feeder vessel from an angular branch of the left MCA was uneventful.
Seven weeks after the second session, a third session of endovascular therapy was initiated. An AVM feeder vessel from a branch of the inferior division of the left MCA was catheterized, and 50 mg amobarbital was injected. (Fig 2B). Focused neurologic examination revealed that strength and sensation in her right arm were intact. With the cognitive portion of the test, her language and visual-perceptual skills remained intact, but she was slightly dysarthric and had considerable difficulty performing calculations. Her arithmetic calculations were 50% correct (three of six answers), compared with 100% correct at baseline and after the previous embolization procedures. Additional tests of right-left orientation were performed, and more arithmetic calculation problems were presented. Right-left orientation remained intact but, initially, the calculation errors persisted. Within approximately 4 minutes, with dissipation of the amobarbital effect, the dysarthria and dyscalculia resolved. At this point, because of the deficits induced, that feeder vessel was not embolized. After repositioning, the microcatheter was positions a few millimeters distally, and another angiogram was obtained (Fig 2C). This revealed the presence of two small branches, which fed the healthy parietal cortex, that were not previously opacified. These vessels were likely correlated with the deficits noted at amobarbital testing. Further embolization sessions were not undertaken, and the patient was referred for subsequent radiation therapy.
Case Illustration 2
The patient was a 52-year-old right-handed woman who began having severe headaches at age 25 years. These were initially treated as migraines, but subsequent brain CT and cerebral angiography revealed a large right parieto-occipital AVM that explained her previously undiagnosed, lifelong, dense, left-sided, homonymous hemianopsia. At age 37 years, she underwent unsuccessful proton-beam therapy. At age 47 years, she underwent uneventful right craniotomy and clip placement in a giant ophthalmic artery aneurysm. She most recently presented with increasing frequency and severity of right-sided headaches, which prompted consideration of further treatment of her AVM.
The patient was scheduled for staged embolization of a large AVM prior to stereotactic radiation therapy. Prior to embolization, she also underwent extensive cognitive examination and underwent a short battery of tests that focused on parieto-occipital functions that was tailored for use during the embolization sessions. During her first embolization session, an amobarbital injection into a right distal posterior cerebral artery (PCA) feeder had no effect on her visual fields (as she had a baseline dense hemifield cut); left motor or sensory function; or ability to perform calculations, read words, or correctly name pictures. However, her ability to correctly identify objects—objects she could specifically identify in her preembolization visit 6 days prior—presented as fragments (11) was impaired (ie, impaired visual object recognition). The vessel was not embolized. The microcatheter was then directed to a different feeder vessel for which provocative amobarbital testing revealed no deficits. This new feeder and two other vessels were embolized during subsequent sessions without incident or resultant deficit.
Discussion
In our experience, tailored neurologic and cognitive testing during AVM embolization procedures is associated with a substantially lower rate of embolization-related neurologic deficits. Compared with previously published reports (7) of studies in which complications occurred in as many as 39% of patients, ours had no hemorrhages and no neurologic deficits in 27 consecutive vessel embolizations in the eloquent cortex. Ethically, we were unable to assess what the functional outcome would have been had we embolized the two vessels in which provocative amobarbital testing induced cognitive deficits. However, embolization of those sites (two [17%] of 12 patients) was unlikely to cause focal cognitive deficits; the result is an embolization-related complication rate well within the previously published range. Some authors have denounced or abandoned amobarbital testing in favor of the decreased potential for motion with general anesthesia (5). Again, our lack of poor outcomes implies that, in experienced hands, tailored provocative testing in awake patients is safe and effective.
The tailored test procedures that we describe are based on both the patient’s individual cognitive abilities and the location of the AVM . They provide brief and easily interpreted results. Knowledge of each patient’s baseline performance with each task is crucial because, frequently, performance in a specific domain is less than that predicted on the basis of patient age and education alone. The AVM itself, other preexisting brain injuries, and/or individual variability, all contribute to deviations from normalized averages. Also to be considered is the possibility of the shifting of functions away from traditional areas of the cortex because of the plasticity related to these chronic lesions (13–17). The possibility of nonstandard functional-anatomic relationships requires that provocative testing include a carefully chosen combination of tasks related to both the precise AVM site and the surrounding regions.
As shown in our case illustrations, inclusion of specific cognitive functions on the basis of the lobe being studied allows detection of potentially disabling deficits beyond those affecting motor, sensory, and speech production. For example, in the cases discussed, tests of only arm strength, sensation, and simple naming and/or reading would have led to embolization and would have resulted in dyscalculia or higher-order visual-spatial deficits after embolization. Previous reports of outcomes and complications related to AVM embolization have graded deficits by categorizing them as major or minor or by using Glasgow Outcome Scale based on the patient’s ability to function independently. Although loss of the ability to independently ambulate, dress, or use the toilet certainly constitutes a major deficit, these gross measures of functional outcome often do not account for losses associated with focal cognitive deficits (18).
Additional advantages with our method is the increased flexibility in microcatheter positioning made possible with provocative testing prior to each potential embolization. During endovascular access of an AVM for embolization with NBCA, an ideal microcatheter position, that is, within the nidus itself, is not always achievable. Factors such as the size, length, and sinuosity of the targeted feeder may limit the progression of the flow-guided microcatheter and require consideration of a more proximal tip position. In such instances, the use of a slower NBCA mixture still allows embolization of the AVM nidus. However, this approach is potentially associated with a higher risk of complications related to the presence of normal distal branches that remain nonopacified during superselective angiography. This was the scenario during the provocative testing described in case 1: the superselective Amytal test provoked dyscalculia, while the preembolization angiogram failed to reveal any normal arterial branches. Although the microcatheter tip could not be advanced more than a few extra millimeters, a second superselective angiogram confirmed the presence of a small distal branch that vascularized healthy cerebral parenchyma.
Cognitive deficits are, by their nature, subtle in their presentation; therefore, their importance is often underestimated when the outcome is established. The lack of dramatic clinical presentation can often hide a cognitive deficit from the unsuspecting physician, or it can delay recognition by the patient herself. For example, impairments in language comprehension or production can have a profound effect on a patient’s ability to interact professionally and socially, and even isolated memory or visual-spatial deficits can undermine a patient’s daily functioning.
Conclusion
Cognitive functions, such as those described, are of paramount importance in a patient’s ability to remain employed, run a household, or safely drive a car (19). The two cases presented involved the provocation of deficits that, had AVM embolization proceeded at those sites, may have been permanent and disabling, as discussed earlier. This method of provocative tailored testing with amobarbital reveals potential deficits, allowing clinicians to make appropriate decisions regarding the risks of each AVM embolization. Widespread use of such a technique by clinicians with formal training in cognitive assessment should help to minimize the potentially disabling outcomes of this invasive and irreversible procedure.
References
- Received May 30, 2001.
- Accepted after revision November 5, 2001.
- Copyright © American Society of Neuroradiology