Abstract
BACKGROUND AND PURPOSE: Qualitative measurement of regional cerebrovascular reactivity (rCVR) to acetazolamide with single-photon emission CT (SPECT) has been widely used to determine the severity of hemodynamic impairment. We attempted to validate the accuracy of qualitative assessment by using SPECT to detect reduced rCVR compared with rCVR determined quantitatively in patients with unilateral major cerebral artery occlusion.
METHODS: Regional cerebral blood flow was assessed with iodine-123-N-isopropyl-p-iodoamphetamine (123I-IMP) at rest and after acetazolamide activation in 133 patients with previously symptomatic, unilateral internal carotid or middle cerebral artery occlusion. Quantitative values were calculated by using the 123I-IMP autoradiographic method and analyzed for each cerebral hemisphere as the percentage change in rCBF between resting and activation studies (%Hem). Qualitative rCVR was determined for the target hemisphere distal to the occlusion as the cerebral-interhemispheric asymmetry index (AIHem) and as an index of flow difference between the target cerebral and ipsilateral cerebellar hemispheres (FIHem−Cbl). Values 2 SDs below the mean in healthy volunteers were defined as decreased.
RESULTS: Fair agreement was observed between %Hem and both AIHem change (resting vs activation, κ = 0.409) and FIHem-Cbl change (resting vs activation, κ = 0.440). When %Hem was assumed to represent the true determinant of assessing rCVR, AIHem change and FIHem−Cbl change demonstrated sensitivities of 68% and 78%; specificities, 72% and 76%; positive predictive values, 48% and 56%; false-positive incidences, 28% and 24%; and false-negative incidences, 32% and 22% for detecting patients with reduced rCVR, respectively.
CONCLUSION: Subgroups of patients with hemodynamic impairment cannot be accurately defined by using rCVR qualitatively measured with SPECT.
Regional cerebrovascular reactivity (rCVR) to acetazolamide, used here as the equivalent of cerebrovascular reserve capacity, is a key parameter in determining the severity of hemodynamic impairment in patients with ischemic cerebrovascular disease. Qualitative measurement of regional cerebral blood flow (rCBF) by using single-photon emission CT (SPECT) with technetium-99m–labeled hexamethylpropyleneamine oxime, technetium-99 m-labeled ethyl cysteinate dimmer or iodine-123-N-isopropyl-p-iodoamphetamine (123I-IMP) has been widely used for assessing rCVR (1–14). Recent prospective studies have demonstrated that rCVR to acetazolamide, as determined quantitatively by using xenon-133 SPECT, can be predictive of the outcome of major cerebral arterial occlusive disease (15, 16). However, in a prospective study performed by using qualitative measurement of rCVR to acetazolamide with 123I-IMP SPECT, Yokota et al (17) failed to find an association of hemodynamic failure and stroke risk. Thus, it is possible that the two SPECT methods, rCVR to acetazolamide measured quantitatively and qualitatively, do not help identify the same patients as having hemodynamic compromise and a high risk of recurrent stroke. Yonas and Pindzola (18) and Yonas et al (19) compared quantitative rCBF measurements to qualitative rCBF methods to determine whether qualitative data based on side-to-side ratios adequately identified the same subgroup with reduced rCVR as quantitative measurements. However, they quantified rCBF values by using the stable xenon-enhanced CT method and then converted these values to ratios of rCBF, instead of the qualitative information obtained by using SPECT.
Recently, a simplified technique, the IMP autoradiographic method, which requires only one-point arterial blood sampling and the acquisition of a single static scan, has been developed to quantify rCBF by using 123I-IMP and SPECT (20, 21). The IMP method is based on the two-compartment model for tracer kinetics. The method uses a standard arterial input calibrated by the radioactivity of a single arterial whole blood sample, a standard lipophilic fraction of IMP in whole blood and a fixed distribution volume (Vd) of IMP (20, 21). Previous studies have demonstrated a good correlation between rCVR to acetazolamide measured by positron emission tomography (PET) with H215O and that measured by using the IMP method. The results have shown that the quantitative assessment of rCVR by using the IMP method has high sensitivity and specificity for detecting patients with compromised reserve (22).
The aim of the present study was to validate the accuracy of qualitative assessment by using SPECT for detecting reduced rCVR to acetazolamide. We compared rCVR to acetazolamide determined quantitatively by using the IMP method with that determined qualitatively by using the degree of right-left cerebral hemispheric asymmetry in the rCBF, or the rCBF ratio to the cerebellar cortex, in patients with chronic, unilateral occlusive disease in a major cerebral artery.
Methods
Patients
A total of 133 patients (101 men, 32 women; mean age ± SD, 57 years ± 10; age range, 41–75 years) with unilateral, chronic, major cerebral artery occlusive disease were included in the study. All patients had ipsilateral carotid-territory symptoms for more than 2 months before their entry into the study. These were evident as either transient ischemic attack (n = 70) or minor completed stroke (n = 63). At entry into the study, patients had scores of 0 (n = 81), 1 (n = 34), or 2 (n = 18) on the modified Rankin disability scale. Brain CT or MR imaging and four-vessel cerebral angiography with arterial catheterization were performed more than 2 months after the last ischemic event and before SPECT studies were performed in all patients. Many patients had either a border zone (n = 62) or a lacunar infarction in the basal ganglia or deep white matter (n = 32). No infarctions were observed in the remaining patients (n = 39). Unilateral, vascular occlusive lesions were noted on the trunk of the middle carotid artery (MCA) in 53 patients and the internal carotid artery (ICA) in 80 patients.
Informed consent was obtained from all patients and healthy volunteers, and the study was approved by the ethics committee of our institution.
SPECT Imaging
SPECT studies were performed within 3 days after cerebral angiography by using a ring-type SPECT scanner, a Headtome-SET080 (Shimadzu Corp, Kyoto, Japan), which provides 31 tomographic images simultaneously. The spatial resolution of the scanner with a low-energy, all-purpose collimator is 13 mm full width at half maximum (FWHM) at the center of the field of view, and the section thickness was 25 mm FWHM at the center of the FOV. Image sections were acquired with 5-mm center-to-center spacing parallel to the orbitomeatal line. The images were reconstructed by using the weighted-filtered backprojection technique, in which the attenuation correction was made by detecting the edge of the object. An attenuation coefficient of 0.065 cm−1, a Butterworth filter (cutoff = 0.45 cycles per centimeter; order = 3) and a ramp filter were used for image reconstruction.
The 123I-IMP SPECT study was performed as described previously (20–22). A 1-minute intravenous infusion of 222 MBq of 123I-IMP (5-mL volume) was administered at a constant rate of 5 mL/min, following a 1-minute infusion of physiologic saline at the same rate. Data acquisition began 20 minutes after the initiation of the 123I-IMP administration for a scanning duration of 20 minutes (a midscan time of 30 minutes).
At 10 minutes after the beginning of the IMP infusion, 2 mL of arterial blood was obtained from the brachial artery. The whole-blood radioactivity of 1 mL of each blood sample obtained was measured by using a well counter that was cross-calibrated to the SPECT scanner. The arterial PaO2 and PaCO2 and the blood pH were also measured in the remaining blood samples by using a blood gas-tension analyzer (IL-1303; Instrumental Laboratory, Danville, IL).
Two days after the measurement of the rCBF at resting state, subjects underwent SPECT with an acetazolamide challenge. Acetazolamide (1000 mg) was given intravenously 10 minutes before 123I-IMP administration, and the SPECT study was performed by using the same procedure as for the resting state.
All reconstructed SPECT images were corrected for the radioactive decay of 123I back to the IMP injection start time, normalized by the data collection time, and cross-calibrated to the well-counter system. The rCBF images were calculated according to the IMP autoradiographic method (20). The whole-blood radioactivity count of the single blood sample was referred to the standard input function. The same standard input function at resting state was used in the calculation of rCBF with the acetazolamide challenge (22, 23). The Vd value was assumed to be 35 mL/mL in the calculation of rCBF images, both at the resting state and with the acetazolamide challenge (22, 24, 25).
Blood pressure was measured by means of auscultation twice during each SPECT study. Mean blood pressure values for each SPECT study were averaged to assess changes in mean blood pressure.
Data Analysis
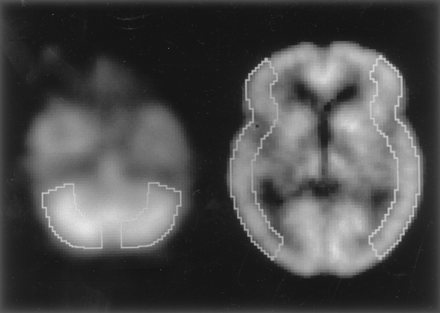
All SPECT images obtained were analyzed as follows. Coregistration and anatomic standardization were performed on a 128 × 128 × 60 matrix (2.25 × 2.25 × 2.25 mm) for each SPECT image by using 3D stereotactic surface projection (3D-SSP, NEUROSTAT) developed by Minoshima et al (26). Supratentorial sections between [anterior commissure − posterior commissure (AC-PC) level − 11.25 mm] and [AC-PC level + 9 mm] and infratentorial sections between [AC-PC level − 38.25 mm] and [AC-PC level − 31.5 mm] of the standardized images that displayed nearly identical cortical shapes were summed together for region-of-interest (ROI) analyses. The outer cortical border was automatically drawn on each section, and the inner cortical border was drawn 15.75 mm (seven pixels × 2.25 mm) further inward on the supratentorial section and 22.5 mm (10 pixels × 2.25 mm) further inward on the infratentorial section. The cortical ribbon on the infratentorial section was defined as the ROI in the cerebellar cortex. The cortical ribbon on the supratentorial section was then divided into 12 30° sectors labeled in a clockwise fashion, and we defined sectors from 30° to 150° as left MCA territory and sectors from 210° to 330° as right MCA territory (Fig 1). One large cortical ROI for each unilateral cerebellar (Cbl) or cerebral (Hem) hemisphere was therefore bilaterally set on each standardized summed SPECT image, and the mean rCBF was determined in each ROI.
ROIs in image sections standardized and summed by using 3D-SSPs.
rCVR to acetazolamide in the MCA territory ipsilateral to the occluded artery was determined on the basis of three methods. For quantitative assessment, %Hem was calculated as the difference in Hem between the value with the acetazolamide challenge and that at the resting state, divided by Hem at the resting state, and then multiplied by 100. Conversely, AIHem was calculated as the interhemispheric difference in Hem divided by the mean value of bilateral Hem values, then multiplied by 100. As the first qualitative assessment, differences between AIHem with the acetazolamide challenge and at the resting state were calculated and defined as AIHem change. Values were positive when rCVR to acetazolamide in the occluded side was higher than that in the contralateral side, and they were negative when rCVR to acetazolamide in the occluded side was lower than that in the contralateral side. Furthermore, relative regional perfusion in the affected MCA territory was also calculated as the ratio of Hem to Cbl ipsilateral to the occluded artery (FIHem−Cbl). As the second qualitative assessment, the difference between the FIHem−Cbl with acetazolamide challenge and at resting state was calculated and defined as FIHem−Cbl change. The value was positive when rCVR to acetazolamide in the affected MCA territory was higher than that in the ipsilateral cerebellum, and it was negative when rCVR to acetazolamide in the affected MCA territory was lower than that in the ipsilateral cerebellum.
Control values of %Hem obtained by using the IMP method from 20 ROIs of 10 healthy volunteers (age range, 30–68 years; mean age, 53 years) were 36.8% ± 9.2 (mean ± SD). Control values of AIHem change obtained from the same volunteers were 0.0 ± 4.2 when the left and right sides were defined as the occluded and contralateral sides, respectively. Control values of FIHem−Cbl change obtained from 20 ROIs of the same volunteers were 0.000 ± 0.040. Values lower than the mean minus 2 SDs of the healthy volunteers (18.4% for %Hem, −8.4 for AIHem change, and −0.080 for FIHem-Cbl change) were defined as decreased.
For statistical analysis, values were expressed as the mean ± SD, and differences between physiologic variables at resting state and with acetazolamide challenge were examined by using a paired t test. Differences between %Hem, AIHem change, and FIHem−Cbl change in patients and healthy volunteers were examined by using the Mann-Whitney U test. The accuracy of AIHem change and FIHem−Cbl change in detecting reduced rCVR was compared by using receiver operating characteristic (ROC) curves. Correlations between two parameters were determined by using linear regression analysis and by computing regression equations and Pearson correlation coefficients. In addition, κ coefficients were computed to assess agreement between two parameters (27). Statistical significance was set at the P < .05 level.
Results
Table 1 shows the mean values of the physiologic variables measured in 133 patients during SPECT scanning at rest and with the acetazolamide challenge. No significant differences in PaO2, PaCO2, blood pH, or mean blood pressure were observed between the two conditions.
Physiologic data in 133 patients measured during SPECT scanning at rest and with an acetazolamide challenge
Table 2 shows the mean values of %Hem, AIHem change, and FIHem−Cbl change in healthy volunteers and patients. All three values were significantly lower in patients than in healthy volunteers.
Values of %Hem, AIHem change, and FIHem−Cbl change in healthy volunteers and patients
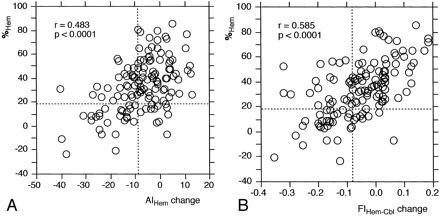
Figure 2 shows comparisons of %Hem with both AIHem change and FIHem−Cbl change to in 133 patients. Significant correlations were observed between %Hem and both AIHem change (r = 0.483; P < .001) and FIHem-Cbl change (r = 0.585; P < .001). Fair κ coefficients were observed between %Hem and both AIHem change (κ = 0.409) and FIHem−Cbl change (κ = 0.440). However, when %Hem was assumed to represent the true determinant (or criterion standard) of assessing reduced rCVR, 27 (28%) of the 96 patients with actual unreduced rCVR had a false-positive AIHem change displaying reduced rCVR. Conversely, 12 (32%) of 37 patients with actual reduced rCVR were false-negatives under AIHem change. Figures 3 and 4 show SPECT rCBF images for two patients identified as having false-positive and false-negative results, respectively. The incidence of false-positive and false-negative results for FIHem−Cbl change obtained by comparing FIHem−Cbl change with the criterion standard %Hem were 24% (23 of 96 patients) and 22% (eight of 37 patients), respectively.
Comparisons of qualitative rCVR with quantitative rCVR (%Hem) calculated by using the IMP autoradiographic method. Significant correlation was observed between these two values. A plot of the relationship between these two values revealed four groups of results: 1) those with reduced rCVR (true-positive), 2) those in which only the quantitative method identified reduced rCVR (false-negative), 3) those without reduced rCVR (true-negative), and 4) those considered reduced rCVR only by the qualitative method (false-positive). Dashed horizontal and vertical lines denote the mean − 2 SDs of the rCVR values obtained in healthy volunteers by using the quantitative method or the qualitative method.
A, AIHem change.
B, FIHem−Cbl change.
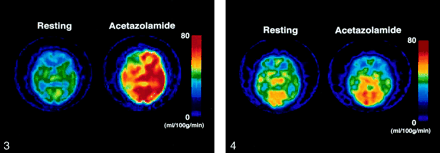
Example of a false-positive result. The same color scale is used to display quantitative rCBF images calculated by using the IMP autoradiographic method at resting state and with the acetazolamide challenge. This patient had a symptomatic occluded ICA on the right side. After the administration of acetazolamide, the patient’s rCBF in the MCA territory increased by 10.3 mL/100 g/min on the right side and by 26.4 mL/100 g/min on the contralateral side. This increase was asymmetrical, with the qualitative assessment defining rCVR as reduced (AIHem change = −28.3), although %Hem on the occluded side, as determined quantitatively, showed a normal flow response of 31.0%.
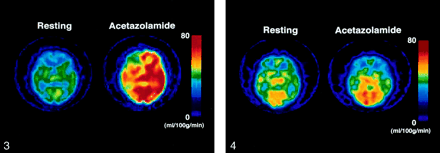
Example of a false-negative result. The same color scale is used to display quantitative rCBF images calculated by using the IMP autoradiographic method at the resting state and with the acetazolamide challenge. This patient had a symptomatic occluded ICA on the left side. After the administration of acetazolamide, the patient’s blood flow in the MCA territory increased only by 2.1 mL/100 g/min on the left side (%Hem = 6.0%) and by 3.1 mL/100 g/min on the contralateral side (%Hem = 8.9%). As asymmetry was absent, the use of rCBF ratios (AIHem change = −2.7) would fail to identify a bilateral reduction of flow response to acetazolamide.
Sensitivity, specificity, and predictive values were calculated for the qualitative assessment of detecting reduced rCVR. AIHem change and %Hem identified the same patients as positive 68% of the time (sensitivity). AIHem change and %Hem identified the same patients as negative 72% of the time (specificity). Patients identified by means of the AIHem change as positive were positive according to %Hem 48% of the time (positive predictive value). Patients identified by means of the AIHem change as negative were negative according to %Hem 85% of the time (negative predictive value). The sensitivity, specificity, positive predictive value, and negative predictive value for FIHem−Cbl change, as obtained by comparison with %Hem, were 78%, 76%, 56%, and 90%, respectively.
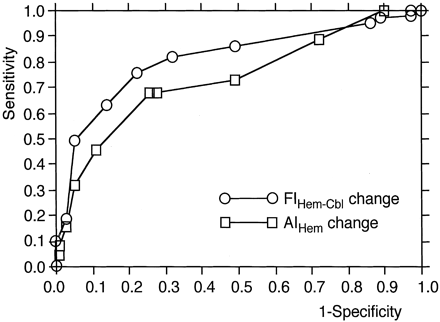
ROC analysis revealed that FIHem−Cbl change was more accurate in detecting reduced rCVR than was the AIHem change (Fig 5). Sensitivity and specificity in the cutoff point lying closest to the left upper corner of the ROC curve were 68% and 74% for AIHem change (cut-off point = −10.0) and 78% and 76% for FIHem−Cbl change (cutoff point = −0.080), respectively.
ROC curves used to determine the diagnostic accuracy of the AIHem change and the FIHem−Cbl change.
Discussion
The present study demonstrated that subgroups of patients with hemodynamic impairment cannot be accurately defined by using rCVR qualitatively measured with SPECT.
Recent prospective studies have demonstrated that rCVR lower than the mean −2 SDs or the 95% confidence lower limit of values quantitatively obtained by using 133Xe SPECT in healthy volunteers is significantly associated with an increased risk of stroke recurrence in patients with symptomatic MCA or ICA occlusion (15, 16). Therefore, in the present study, we defined a %Hem volume as being reduced when it was lower than the mean −2 SDs of values obtained quantitatively by using the IMP method in healthy volunteers; that is, this value indicated those at high risk of stroke recurrence. When the accuracy of the IMP method for quantifying rCVR to acetazolamide is validated by comparing it to rCVR determined quantitatively by using PET with H215O, the IMP method reportedly has 90% sensitivity and 92% specificity for detecting patients with reduced rCVR (22).
Proponents of qualitative methods for studying rCVR have suggested that comparison of right-left cerebral hemispheric asymmetry in the rCBF, or the rCBF ratio to the cerebellar cortex before and after acetazolamide, can provide an objective evaluation of hemodynamic effects (1–14). Our data indicate that, although a fair agreement existed between %Hem and both AIHem change and FIHem−Cbl change, qualitative assessments had only 68–78% sensitivity and 72–76% specificity for detecting patients with rCVR. Positive predictive values determined by using qualitative data were only 48–56%, and negative predictive values were 85–90%. The incidences of false-positive results (ie, classifying patients as being at increased risk when they did not actually fulfill the criteria according to quantitative methods) was 24–28%, and the rate of false-negative results was 22–32%. These results suggest that subgroups of patients at increased risk for stroke recurrence cannot be accurately defined according to rCVR qualitatively measured by using SPECT. Our results correspond with those of reports by Yonas et al (19) obtained by using stable xenon-enhanced CT. Their qualitative assessment had 61% sensitivity, 75% specificity, and a 50% positive predictive value for detecting patients with compromised reserves. They concluded that qualitative assessments of rCVR are not specific enough to guide management in patients with symptomatic carotid occlusive disease.
A number of factors may contribute to low sensitivity and specificity of qualitative assessment for detecting patients with reduced rCVR. Results whereby patients with negative values of AIHem change have normal %Hem may occur when rCBF increases asymmetrically after the administration of acetazolamide. Despite the fact that the cerebral hemisphere ipsilateral to the occluded artery exhibits normal quantitative values of the rCBF response to acetazolamide, qualitative assessments such as AIHem change may incorrectly identify ipsilateral cerebral hemodynamics as displaying significant compromise (false-positive) when the rCBF response to acetazolamide in the contralateral cerebral hemisphere is higher (to some extent) than that in the ipsilateral hemisphere. Conversely, patients with unilateral ICA occlusion, particularly those with collateral circulation through the anterior communicating artery, may have a hemodynamic disturbance in both cerebral hemispheres (28, 29). Furthermore, mild and diffuse arteriosclerosis may impair cerebral hemodynamics on the contralateral side despite the presence of angiographic data indicating no lesions (19, 29). In these two situations, the cerebral hemisphere ipsilateral to the occluded artery may be identified as displaying normal hemodynamics (false-negative) when values of the contralateral hemisphere are used as internal controls. When the rCBF response to acetazolamide in the cerebellar hemisphere is higher than that in the cerebral hemisphere ipsilateral to the occluded artery with normal quantitative values of rCVR or when mild and diffuse arteriosclerosis impairs cerebellar hemodynamics, qualitative assessment by using values from the cerebellar hemisphere as internal controls may likewise result in false-positive or false-negative results, respectively. However, because ROC analysis in the present study revealed that FIHem−Cbl change was more accurate in detecting reduced rCVR than AIHem change, the aforementioned factors may occur less frequently in the cerebellar hemisphere than in the cerebral hemisphere.
The methods of rCBF measurement used in the present study possess certain limitations worthy of discussion. SPECT studies at the resting state and with the acetazolamide challenge were performed 2 days apart in all patients. This separation of the two SPECT studies represents an important problem, as many physiologic parameters may have changed between studies. However, no significant differences in PaO2, PaCO2, blood pH, or mean blood pressure were identified between studies; therefore, the observed rCVR was unlikely to have been influenced by physiologic changes.
Conclusion
Qualitative assessments of rCVR can be performed by using any SPECT scanner and do not require arterial blood sampling. As a result, the technique can be readily used for assessing rCVR in routine clinical practice. However, the results of the present study suggest that the risk of stroke recurrence may not be assessable with the qualitative measurement of rCVR by using SPECT in patients with symptomatic, major cerebral artery occlusive disease.
Acknowledgments
The authors wish to thank Professor Satoshi Minoshima, University of Washington Medical School, for both the use of 3D-SSP (NEUROSTAT) and for his helpful suggestions.
Footnotes
This work was supported in part by Grants-in-Aid for Advanced Medical Scientific Research by the Ministry of Science, Education, Sports and Culture, Japan.
References
- Received October 28, 2002.
- Accepted after revision January 27, 2003.
- Accepted after revision January 27, 2003.
- Copyright © American Society of Neuroradiology