Abstract
BACKGROUND AND PURPOSE: Routine carotid sonography and MR angiography cannot reliably detect the markedly reduced flow velocities associated with very severe carotid stenosis. In this study, we sought to evaluate the accuracy of single row detector helical CT angiography in distinguishing hairline residual lumen from total occlusion of severely stenosed internal carotid arteries (ICAs).
METHODS: From our departmental data base of single row detector CT angiography studies performed for evaluation of ICA occlusive disease, 21 cases were identified with evidence of either hairline residual lumen or total occlusion on correlative conventional catheter radiographic arteriograms; these included seven cases of proved hairline residual lumen and 14 cases of proved total occlusion. Two neuroradiologists, blinded to the radiographic arteriography results, graded the diseased ICA on each CT angiogram as definitely occluded, probably occluded, indeterminate, probably patent, or definitely patent. Receiver operating characteristic curves were generated for each neuroradiologist.
RESULTS: At an operating point on the receiver operating characteristic curve corresponding to 90% sensitivity, the first reader achieved 95% specificity and the second reader achieved 80% specificity for distinguishing hairline residual lumen from total occlusion. Absolute accuracy rates were 95% and 85%, respectively. No significant difference in accuracy was observed between the two readers (P = .28, two-tailed t test).
CONCLUSION: Single row detector CT angiography can distinguish total ICA occlusion from hairline residual lumen with a high degree of accuracy. In equivocal cases, conventional catheter arteriography may be desirable to confirm the diagnosis.
Stroke continues to be a leading cause of morbidity and mortality in the United States. Symptomatic stenosis of the internal carotid artery (ICA) of >70% is correlated with significantly increased risk, typically requiring carotid endarterectomy rather than medical treatment (1–3). ICA stenosis in the extreme has been variously designated as carotid pseudo-occlusion, near occlusion, string sign, or hairline residual lumen by some authors (4, 5). Hairline residual lumen can be defined as an extremely narrowed lumen (>90%–99% stenosis), associated with turbulent flow and diminished flow velocities (4–6). The differentiation between true total occlusion and hairline residual lumen is sometimes essential in that carotid endarterectomy or stent placement may be considered for cases of hairline residual lumen but not typically for cases of complete occlusion (3, 7). Moreover, routine sonography and unenhanced MR angiography may be inadequate to detect the markedly diminished flow velocities associated with hairline residual lumen (hence the term pseudo-occlusion). The “gold standard” for diagnosis, therefore, remains conventional catheter radiographic arteriography, with its associated risks, including stroke (5, 8–10).
Several studies have been conducted comparing the accuracy, sensitivity, and specificity of CT angiography versus unenhanced MR angiography, radiographic arteriography, and ultrasonography in the assessment of atheromatous stenosis of the extracranial carotid arteries (9, 11–16). These studies, however, have not emphasized the ability of noninvasive carotid imaging to discriminate between hairline residual lumen and total occlusion in that subgroup of patients with extreme ICA stenosis. Some authors have grouped hairline residual lumen in the same category as total occlusion for their data analysis (11, 17, 18). Furthermore, studies that have made a distinction between hairline residual lumen and total occlusion reveal that unenhanced MR angiography may misclassify <70% of severely stenosed ICAs as occluded (14, 19). Although more recent investigations, such as that conducted by El-Saden et al (20), report better discriminatory ability for both ultrasonography and unenhanced MR angiography, these modalities remain limited in that ultrasonography is highly operator dependent and significant contraindications to MR imaging exist (including difficulty imaging critically ill ventilator- or pressor-dependent patients). In this study, we compared the sensitivity and specificity of single row detector helical CT angiography with those of conventional catheter arteriography in the differentiation of hairline residual lumen from complete ICA occlusion.
Methods
From our departmental data base of single row detector CT angiography studies performed for evaluation of ICA occlusive disease, 21 cases were identified with evidence of either hairline residual lumen or total occlusion on correlative conventional catheter radiographic arteriograms. Seven of these 21 patients had arteriographically confirmed hairline lumina; the remaining 14 had proved total occlusion. Additional correlative 2D time-of-flight MR angiograms (4) or ultrasonograms (3) were available for six of the hairline residual lumen cases; one of the patients with hairline residual lumen underwent all four imaging modalities. Correlative 2D time-of-flight MR angiograms (8) or ultrasonograms (7) were available for 11 of the total occlusion cases; four of the patients with total occlusion underwent all four imaging modalities.
CT Angiography
All CT angiography was performed by using a single detector row helical scanner (either a GE High Speed Advantage or GE CTi; GE Medical Systems, Milwaukee, WI). The patient’s head was placed in a headholder, and unenhanced axial view CT of the neck was performed from approximately the C5−C6 level to the skull base to identify the level of the carotid bifurcations, assess for calcifications, and adjust scan angle to minimize metallic dental artifact through the ICA. Scan parameters included 5-mm-thick axial sections, 120 kV, 170 mA, and 2-s scanning time (340 mAs). Helical CT angiography was performed immediately after unenhanced CT, scanning from just below the level of the bifurcation to the skull base, by using the following parameters: pitch, 1; collimation, 3 mm; maximal mA (limited by tube heating, typically 210–250 mA), kV 140; field of view, 18 cm; and 90–120 mL of nonionic contrast material (administered by power injector at 2–3 mL/s into an antecubital fossa vein) (Medrad, Indianola, PA) with a fixed 25-s delay between the onset of contrast material injection and the start of scanning (the delay was increased to 40 s for patients with atrial fibrillation). The resulting 3-mm-thick contiguous axial view sections were reformatted into 1-mm-thick scans by the CT technologist, which were digitally archived together with the 3-mm source scans.
Retrospective CT angiogram review was performed at a PACS workstation (AGFA Impax RS3000 1K review station; AGFA Technical Imaging Systems, Richfield Park, NJ) by two board-certified neuroradiologists experienced in carotid CT angiography interpretation. The neuroradiologists were blinded to the clinical history, lateralization of symptoms, and radiographic arteriography results but were aware of the suspicion for hairline residual lumen versus total occlusion. Patient name, age, sex, and date of examination were electronically masked during scan review. Neither reader had participated in the selection of the cases or in the analysis of the correlative imaging findings. Care was taken to use appropriate window and level settings during scan review to optimize visualization of intraluminal CT contrast attenuation, compared with that of the surrounding soft tissues and calcifications in the vessel wall (21).
Scan review was primarily of the thin section axial view source scans at a PACS workstation. Specifically, scan interpretation was accomplished by scrolling through both the 3-mm-thick axial view source scans and the 1-mm-thick axial view reformatted scans, at varying zoom settings, bi-directionally from the distal common carotid artery to the distal intracranial ICA. Hairline residual lumen was diagnosed if the reader was able to follow a narrow or punctate intraluminal column of contrast material along consecutive axial view scans (Fig 1A–D). 2D and 3D vascular reconstructions, including curved reformatted scans, created and digitally stored by dedicated technologists at our institution’s 3D laboratory, were additionally examined when available but were not relied on for case interpretation, secondary to the intrinsic subjectivity and operator dependence involved in their construction (Fig 2B). All diseased ICAs were independently rated by both readers according to the following 5-point scale: 1, definitely occluded (total occlusion); 2, probably occluded; 3, possibly occluded (indeterminate); 4, probably patent; and 5, definitely patent (hairline residual lumen). During data analysis, vessels with rating scores of 1 or 2 were categorized as “occluded,” whereas those with rating scores of 4 or 5 were categorized as “hairline.”
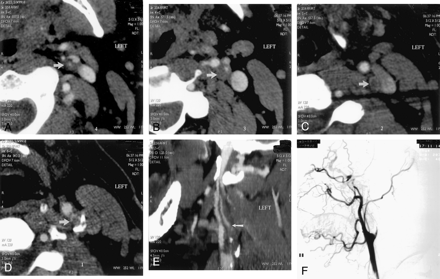
A−D, Selected axial view source scans from a CT angiogram of a 74-year-old female patient show continuity of the contrast-filled lumen (arrows) in consecutive axial views of the right ICA, reflecting a patent vascular lumen. Scans progress from inferior to superior levels, beginning at the patent carotid bifurcation. Note that in A, the jugular vein, posterior and lateral to the carotid artery, has not yet opacified.
E, Sagittal view curved reformatted projection of this CT angiography dataset shows a hairline residual right ICA lumen with a slim sign extending into the petrous canal at the skull base. Note that the vessel appears falsely discontinuous along portions of the scan, which is a potential pitfall of this postprocessing technique, emphasizing the need for review of the axial view source scans. ICA origin is seen just superior to a discontinuous segment (arrow).
F, Lateral view digital subtraction arteriogram obtained during injection of the right common carotid artery confirms critical stenosis of the right ICA origin, with diffuse narrowing (hairline residual lumen) of the more distal right ICA.
A−D, Selected source scans from a CT angiogram of a 45-year-old man. Unlike those shown in Figure 1, these scans reveal discontinuity of the intraluminal left ICA contrast column, reflecting an occluded vessel (arrow in A indicates residual ICA lumen; arrows in B−D indicate unopacified ICA lumen/enhancing vasa vasorum). Scans progress from inferior to superior levels.
E, Curved reformatted projection constructed from this CT angiography dataset shows left ICA occlusion, with absent intraluminal contrast opacification. An opacified ascending pharyngeal artery (arrow) mimics a patent left ICA but cannot be followed into the petrous canal.
F, Lateral view digital subtraction arteriogram obtained during injection of the left common carotid artery confirms total occlusion of the proximal left ICA.
Conventional Catheter Radiographic Arteriography
Carotid radiographic arteriography was performed by using nonionic contrast material as per standard clinical practice described by Morris (22). In all instances, imaging was continued into the late venous phase to assess delayed filling related to severely stenotic ICAs. Two neuroradiologists experienced in the interpretation of radiographic arteriography individually interpreted the scans; the vessels in question were classified as either occluded or showing patency with a hairline residual lumen. Results were compared both between readers and with the official clinical reports generated by the staff neuroradiologists who had performed the arteriograms; complete agreement was achieved for all cases without interobserver variation.
Data Analysis
Conventional arteriography was considered to be the gold standard test, reflecting the true grade of stenosis. Sensitivity and specificity for differentiating hairline residual lumen from total occlusion were calculated for CT angiography at various cut points for each reader, generating receiver operating characteristic curves.
Results
No complications occurred during the administration of contrast material for either CT angiography or radiographic arteriography in this group of patients. All examinations were of diagnostic quality.
The mean ages of the hairline residual lumen and total occlusion patient groups were 72.1 ± 7.7 and 66.5 ± 10.1 years, respectively. The average age of the entire patient pool was 69.0 ± 9.5 years (range, 48–81 years). The difference in mean ages between the two patient groups was not statistically significant (P = .22, two-tailed t test). The total occlusion group consisted of 11 men and three women; the hairline residual lumen group consisted of four men and three women.
For the hairline residual lumen group, the timing of the CT angiography examination ranged from 13 weeks before to 5 weeks after radiographic arteriography (Fig 1C). On average, a 6.5-day delay occurred between performance of CT angiography and subsequent conventional arteriography. For the total occlusion cases, the timing of the CT angiography examination ranged from 4 weeks before to 7 years after radiographic arteriography (Fig 2C). On average, a 38-week delay occurred between performance of conventional arteriography and subsequent CT angiography.
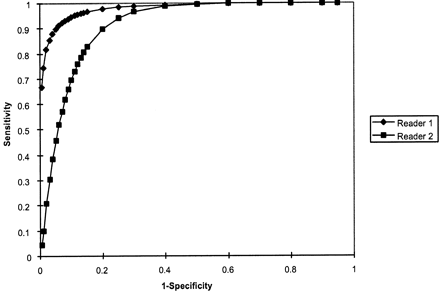
At an operating point on the receiver operating characteristic curve corresponding to 90% sensitivity for CT angiography detection of hairline residual lumen, reader 1 achieved 95% specificity and reader 2 achieved 80% specificity. Absolute accuracy rates were 98% and 91%, respectively. No significant difference was observed in accuracy between the two readers (P = .28, two tailed t test) (Fig 3). Significant categorical interobserver disagreement between the hairline residual lumen and total occlusion classification scores, based on the CT angiography findings, was present for only two cases (Table 1). In one total occlusion case, reader 2 misinterpreted an ascending pharyngeal artery as a patent hairline ICA (Fig 2). Reader 1 interpreted a different hairline residual lumen case as probably occluded. In no other instance did the readers disagree in classifying a vessel as definitely/probably patent versus definitely/probably occluded. Thus, overall, categorical agreement existed between the two readers for 91% of the 21 cases (Table 2).
Receiver operating characteristic curves for detection of hairline residual lumen by readers 1 and 2. Area under the receiver operating characteristic curve for reader 1 (which correlates with accuracy) was 0.98 ± 0.03; for reader 2, this area was 0.91 ± 0.06. No significant difference was observed between the accuracy of the two readers (P = .28, two-tailed t test). Of note, at an operating point corresponding to 90% sensitivity, reader 1 achieved 95% specificity and reader 2 achieved 80% specificity. At an operating point corresponding to a sensitivity of 95%, specificity was reduced to approximately 90% for reader 1 and 75% for reader 2.
CT angiography reader ratings*
Inter-rater variability*
Discussion
Hairline residual lumen, also referred to as carotid artery pseudo-occlusion or near occlusion, is defined as a markedly narrowed ICA lumen, accompanied by turbulent slow flow (3, 4). This is typically associated with focal, severe stenosis at the ICA origin, with an extremely reduced poststenotic pulse pressure and therefore collapse of the muscular arterial wall of the distal vessel. The differentiation of pseudo-occlusion from true total occlusion has important implications for both prognosis and treatment. For patients with hairline residual lumen, stroke risk will be reduced after carotid endarterectomy (23, 24). Patients with preexisting complete occlusion, however, are typically unlikely to benefit from surgery. Masaryk and Obuchowsky (25) have noted that a carotid artery imaging technique should be able to accurately quantify the degree of stenosis, distinguish between severe stenosis and occlusion, and detect associated abnormalities that could affect the surgical procedure (such as vascular loops). It could be argued that carotid CT angiography meets these clinical requirements. The objective of our study was to determine the accuracy of single detector row helical CT angiography, compared with that of conventional catheter arteriography, in distinguishing hairline residual lumen versus total occlusion of the extracranial ICA.
Although several authors (26–29) have reported that unenhanced MR angiography is efficacious in diagnosing carotid occlusion, most such studies have not emphasized the distinction between pseudo- and total occlusion. Multiple studies have emphasized, however, that time-of-flight MR angiography may overestimate the degree of ICA stenosis; some of these studies have also noted a lack of sensitivity in differentiating hairline residual lumen from true occlusion (8, 10, 26, 30, 31). Riles et al (19), for example, reported failure to detect flow in 70% of pseudo-occluded ICAs. Furst et al (10) showed that 2D time-of-flight MR angiography misclassified 30% and that 3D time-of-flight MR angiography misclassified 53% of ICA pseudo-occlusions as complete occlusions. In a review article, Bowen et al (30) state that 2D time-of-flight MR angiography may overestimate severe stenoses, with complete loss of signal intensity on 2D time-of-flight projection MR angiograms, in as many as 85% of carotid arteries with stenoses >70% to 80%. Although the accuracy of 2D time-of-flight MR angiography in distinguishing hairline lumen from occlusion was not specifically addressed by our study, it is noteworthy that, anecdotally, all our hairline lumen cases for which correlative 2D time-of-flight MR angiograms were obtained (four of seven cases) showed complete signal intensity dropout.
Contrast-enhanced MR angiography shows great promise in overcoming many of the limitations of 2D time-of-flight MR angiography, and although it is gaining rapid acceptance as a clinical tool, it has not yet been as thoroughly validated as CT angiography in cases with the greatest degree of stenosis (32–35). At least one author has commented that contrast-enhanced MR angiography alone has not proved accurate enough to routinely replace radiographic arteriography in carotid artery evaluation (35). In another study, severe ICA stenoses were detected with 100% sensitivity and specificity by CT angiography and with 93% sensitivity and 100% specificity by contrast-enhanced MR angiography. Although intraluminal surface irregularities were more frequently detected by CT angiography, both modalities revealed more ulceration than was detected by radiographic arteriography alone (34).
Our study indicated that helical CT angiography facilitates accurate differentiation of ICA pseudo-occlusion from true ICA occlusion. Categorical agreement with conventional arteriography existed, for both readers, in 91% of our cases, without significant difference in accuracy between the two readers (P = .28). CT angiography can be performed on an outpatient basis, takes only a few minutes to complete, and is minimally invasive. With single detector row helical scanners, the circle of Willis can additionally be evaluated during a carotid CT angiography examination. In theory, because our CT angiography protocol calls for a uniform 25-s “prep” delay before the start of scanning, one might even anticipate CT angiography to be more reliable than conventional arteriography in distinguishing hairline residual lumen from total occlusion because of a more prolonged scanning time, which increases the time available for contrast material to opacify potentially slow flow within a hairline residual lumen. Moreover, both our radiographic arteriography and CT angiography protocols typically require the acquisition of delayed scans when there is suspicion for vascular occlusion during real time examination monitoring, to more confidently exclude a false positive diagnosis of occlusion in cases with very slow flow. Other limitations of CT angiography include its contraindication in patients with renal function impairment or a history of adverse reaction to contrast material. Another potential disadvantage of CT angiography is miscalculation of the degree of stenosis due to the presence of heavily calcified plaque, although this problem was not encountered in our study (31).
Our scan review procedure for carotid CT angiography involves scrolling through the axial view source and reformatted scans at a digital workstation, by using maximal and variable zoom of the carotid arteries, from the level of the distal common carotid artery to the petrous ICA at the skull base. Additional 2D and 3D reformatted scans (created by technologists at our dedicated 3D imaging laboratory) were reviewed as well, but were considered to be less reliable for distinguishing hairline residual lumen from total occlusion, because postprocessing may introduce distortions of the dataset. It is important to follow the course of the distal extracranial ICA into the petrous canal at the skull base, to avoid the pitfall of confusing the ascending pharyngeal branch of the external carotid artery (which does not enter the skull) with a patent, hairline ICA.
Both this study and our clinical experience suggest that visual integration by “connecting the dots,” as it were, of intraluminal ICA contrast material along consecutive, contiguous axial view source scans is the key to an accurate diagnosis of a hairline residual ICA lumen (Fig 1A–D, arrows). In cases for which sequential scan review fails to reveal a continuous column of intraluminal contrast material but rather shows a discontinuous, heterogeneous matrix of contrast material-filled “dots,” the vessel in question is likely occluded (Fig 2A–D, arrows). For such cases, which were uncommon in our series, we continue to recommend further evaluation with conventional arteriography to our referring clinicians. If no intraluminal ICA contrast material is identified on multiple contiguous axial view sections, however, we consider the vessel to be occluded and the imaging workup to be complete. The presence of noncontinuous “dots” of contrast material along the periphery of the vessel wall may reflect enhancement of the vasa vasorum, which has been reported to potentially mimic a slim sign (36).
Finally, it should be noted that the data for this study were collected by using older, single detector row helical CT scanners. Although our current clinical practice suggests that the accuracy for multi-detector row helical CT scanners to distinguish between hairline and occluded ICAs may be even greater than that reported herein, the low number of conventional catheter arteriograms that we now obtain renders future studies using radiographic arteriography as a gold standard impractical.
Conclusion
Single detector row helical CT angiography can accurately distinguish hairline residual lumen from total occlusion of the ICAs. In cases in which the classification remains uncertain, conventional catheter arteriography may be useful to confirm the diagnosis.
Footnotes
A preliminary version of this work was presented at the 35th Annual Meeting of the American Society of Neuroradiology, Toronto, Canada, 1997.
This work was partially supported by an educational grant from GE Medical Systems.
References
- Received May 10, 2002.
- Accepted after revision January 2, 2003.
- Accepted after revision January 2, 2003.
- Copyright © American Society of Neuroradiology