Abstract
Summary: Direct percutaneous puncture for coil placement has been described for visceral aneurysms, but the procedure has not been previously reported for aneurysms of the head and neck. We report a case in which stent-assisted endovascular treatment was successfully combined with direct puncture for additional coil placement to treat a symptomatic giant aneurysm of the cervical internal carotid artery.
Pseudoaneurysms of the cervical internal carotid artery (ICA) are uncommon lesions that may be induced by trauma, dissection, or rarely, infection or tumor invasion (1–4). Open surgery for the treatment of such lesions has been partly replaced by endovascular treatment alternatives such as balloon occlusion, coil embolization, stent placement, and balloon- or stent-assisted coil embolization (1–3, 5–8). The last technique has found widespread application in the treatment of wide-necked intra- and extracranial aneurysms, especially in recent years (1–3,6–8).
Direct percutaneous puncture and embolization of lesions such as arteriovenous malformations and hypervascular tumors has been described for the head and neck region (9, 10). Absolute alcohol, polyvinyl alcohol particles, gelatin foam pledgets, n-butyl cyanoacrylate, and coils have all been used for percutaneous and transarterial embolization of these lesions (9, 10).
Direct percutaneous puncture and embolization has been used in visceral (11) and peripheral (12) aneurysms, but the procedure has not been reported in the treatment of pseudoaneurysms of the ICA. Here we report a case of a cervical ICA dissecting aneurysm that was treated with a combination of stent-assisted coil placement followed by direct percutaneous puncture and further placement of Guglielmi detachable coils (GDC; Target Therapeutics, Fremont, CA).
Case Report
An 80-year-old woman presented to the emergency room with an enlarging mass in the back of her mouth that was interfering with her swallowing. She had first noticed this mass 3 months earlier; it had slowly grown to its present size without causing any bleeding or pain. The clinical history revealed a previous minor stroke affecting the right side of her upper extremity; the symptoms had significantly improved. MR imaging of the neck showed a large dissecting aneurysm in the left parapharyngeal region extending into the oral cavity.
The patient was emaciated and denied any history of trauma, infection, or other disease. On physical examination, a pulsatile mass was found in the left superior-posterior oral cavity originating from the region of the oropharynx and extending toward the hypopharynx. Neurologic examination showed a mild weakness of the right upper extremity (4/5) and intact cranial nerves. Blood chemistry and cultures were unremarkable; sedimentation rates were within normal limits.
Surgery was not considered an option, because of the patient’s advanced age and poor health condition. She was referred to us for a diagnostic angiogram and potential endovascular treatment.
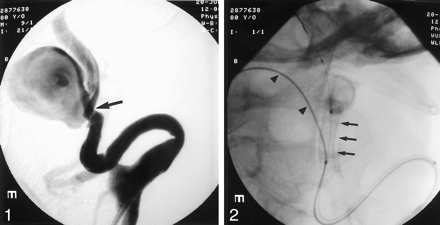
Angiograms showed a very tortuous anatomy and a giant dissecting aneurysm of the left cervical ICA of 26 × 27 × 32 mm with a 10-mm-wide neck. The distal ICA measured 4.4 mm. Severe kinking at the origin of the ICA and stenosis resulting from dissection were found at the proximal neck of the aneurysm (Fig 1). Before intervention, 5000 U of heparin was administered; the activated clotting time was maintained between 250 and 300 seconds. Balloon test occlusion (BTO) was performed to determine whether carotid obliteration was a treatment option should stent-assisted embolization fail. Neurologic examination and a single-photon emission CT (SPECT) study with Tc99 m ECD (Neurolite; E.I. Dupont de Nemours, Billerica, MA) for cerebral perfusion were performed.
Selective left carotid angiogram shows a giant dissecting aneurysm of the cervical ICA. The neck of the aneurysm measures 10 mm. Stenosis and wall irregularities of the ICA are at the level of the aneurysm neck (arrow).
BTO was negative, although the perfusion scans showed a 50% reduction in left hemispheric perfusion during occlusion. An existing fetal variant of the left posterior cerebral artery may have contributed to the global reduction of flow to the left hemisphere during the BTO.
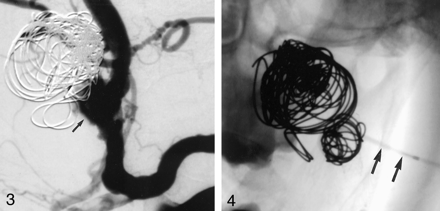
Stent-assisted GDC embolization of the dissecting aneurysm was initiated. With the patient under general anesthesia, a 7F Vista Brite Tip guiding catheter (Cordis, Miami Lakes, FL) was advanced into the left ICA over an Amplatz superstiff guidewire (William Cook Europe, Bjaeverskov, Denmark); a roadmap was created. A 0.014-inch ACS Hi-Torque Balance guidewire (Guidant, Temecula, CA) was manipulated into the distal ICA, and a separate Agility 0.014-inch soft wire (Cordis) was navigated into the aneurysm. A Prowler Plus microcatheter (Cordis) was advanced over the Agility wire into the aneurysm. A 4 × 15-mm S 7 balloon-expandable stent (Medtronic, Santa Rosa, CA) was brought over the Balance wire to the level of the aneurysm neck (Fig 2). The stent was deployed, jailing the microcatheter inside the aneurysm and crossing the neck of the lesion. The ICA remained patent. In an attempt to reduce vortex flow inside the aneurysm and help create stasis and thrombus formation (1, 8), a second stent of the same size was deployed over the first stent, reducing the porosity of the stents. Numerous GDCs (Target Therapeutics, Fremont, CA) were then deployed into the aneurysm sac via the microcatheter (Fig 3). After most of the dome was coiled and flow stasis was achieved, the microcatheter inadvertently slipped out of the aneurysm sac. Multiple attempts to renavigate the microcatheter into the aneurysm through the stent failed. A control angiogram showed a patent ICA with decreased intraaneurysmal flow and areas of filling defect (Fig 3). We terminated the procedure, hoping to achieve spontaneous complete thrombosis after discontinuation of heparin. The patient was discharged the next day with instructions to take 325 mg aspirin and 75 mg Clopidogrel by mouth q.d.
The stent is positioned across the neck of the aneurysm jailing the microcatheter, which has been placed inside the aneurysm (arrowheads).
Follow-up arteriogram acquired 1 month after the initial procedure shows most parts of the aneurysm thrombosed, but the neck is patent (arrow).
Sonography performed 4 weeks after the procedure showed that the neck of the aneurysm and the ICA were still patent. Although the mass had reduced in size, the patient expectorated bloody sputum once.
We decided to treat the remaining aneurysm. With the patient under general anesthesia and full heparinization (activated clotting time, 250–300 seconds), we once again attempted to place a microcatheter into the aneurysm through the stent but failed. A direct percutaneous puncture of the aneurysm was undertaken. Leaving the guiding catheter inside the origin of the left ICA, the patient’s neck was prepped and draped in a sterile fashion. From a high posterior submandibular approach, an 18-gauge coaxial needle system was used to puncture the neck of the aneurysm under fluoroscopic guidance, while contrast was injected into the ICA to navigate the percutaneous approach (Fig 4).
Radiograph, anteroposterior projection, shows a needle placed percutaneously into the aneurysm (arrow). A microcatheter has been navigated through the needle for coil placement.
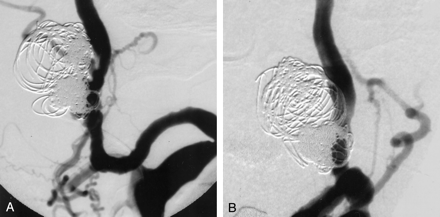
After appropriate positioning and stabilization of the needle with an anchor suture and Tegaderm (3M; Health Care, St. Paul, MN), continuous heparinized saline flush was established. A Prowler Plus (Cordis) microcatheter was placed through the needle into the aneurysm, and GDCs were packed until the aneurysm was completely occluded and the ICA remained patent (Figs 4 and 5).
Angiograms in lateral (A) and frontal (B) views acquired following percutaneous coil placement show no filling of the aneurysm with patent ICA. Note some coils are projecting into the stented segment.
Heparin was reversed with 20 mg of intravenous Protamine. The needle and catheters were withdrawn; manual compression over the puncture site was applied, and hemostasis was easily achieved. The patient was extubated the next morning, and further reduction in the diameter of the mass in her oral cavity was noted. Early follow-up sonography showed no blood flow inside the aneurysm, while the left stented ICA remained patent. The patient was discharged with instructions to take 325 mg aspirin and 75 mg Clopidogrel by mouth q.d. At the 3-month clinical follow-up, the patient remains asymptomatic, and sonography findings are unchanged.
Discussion
Aneurysms of the cervical ICA are rare (3, 4). Clinical sequelae of cervical ICA aneurysms result from ischemia secondary to luminal narrowing, thromboembolism, and compression of adjacent nerves causing lower cranial neuropathy or Horner syndrome (1). Untreated, such aneurysms are associated with significant morbidity and mortality of up to 80% and 40%, respectively (1). Onset of symptoms may be delayed by months or years, and patients may not recall the initiating event, as in our case. Surgical treatment involves complete aneurysm resection with or without interposition grafting (2). This, however, may be associated with a high mortality rate of up to 30% (2) and a high incidence of cerebral complications. Surgical repair may be limited by the inaccessibility of lesions close to the skull base (3, 4).
Recent advances in endovascular technology have allowed safer treatment options for these lesions (1–3, 5–8). Stent-assisted coil embolization of intracranial and extracranial aneurysms has evolved into a preferred technique of treatment for wide-necked aneurysms (1–3, 6–8). The deployment of a self- or balloon-expandable stent across the neck of an aneurysm causes flow alteration within the aneurysm, which may lead to stable thrombus formation (1, 8). The stent also serves as a scaffold for tighter packing of a wide-necked aneurysm with coils (1, 3, 6–8). In our case, the neck of the dissecting aneurysm measured 10 mm with adjacent stenosis and vessel wall irregularities. The goal of the stent-assisted coil placement was to provide support at the neck, reconstruct the diseased arterial segment, and maintain patency. Because the BTO with SPECT CT study revealed an ipsilateral CBF reduction, we did not attempt coil placement without stent assistance.
Incomplete occlusion of the aneurysm after the initial coil treatment and the remaining risk of life-threatening hemorrhage necessitated a second approach that involved direct percutaneous puncture. An earlier attempt at accessing the aneurysm via the endovascular route had failed.
Direct percutaneous puncture and embolization has also been practiced with increasing frequency in the treatment of peripheral and visceral aneurysms, especially when they are inaccessible via the endovascular route (11, 12). This approach has been used for a variety of head and neck diseases, including hypervascular tumors (9) and arteriovenous malformations (10). To our knowledge, however, a direct percutaneous coil placement in aneurysms of the cerebral vasculature has not been reported. Potential complications such as hemorrhage, air and thromboembolism, displacement of coils, or seepage of liquid embolic agents into vital structures explain the reluctance of interventionalists to use this approach.
The use of a microcatheter through the needle for coil placement allows an appropriate working distance from the radiation source and also provides additional stability to the system during coil deployment.
Conclusion
Unlike liquid embolic agents, coils can be placed in a more controlled fashion. Overall, direct percutaneous embolization may be a feasible alternative or adjunct to endovascular therapy of cervical ICA aneurysms, especially when they are not accessible by the transarterial route. Use of smaller gauge needles, continuous flush, and coils made this approach safe and effective in our case. This technique needs to be evaluated in a large group of patients before it can be accepted as a standard of care.
Acknowledgments
We wish to thank Dagmar Schnau for her editorial assistance.
Footnotes
Address correspondence to Dagmar Schnau, M.F.A., Editorial Office, Section of Neuroendovascular Surgery and Interventional Neuroradiology, Department of Radiology, University of Miami of Miami School of Medicine, 1611 N.W. 12th Avenue, WW279, Miami, FL 33136.
References
- Received July 29, 2002.
- Accepted after revision November 3, 2002.
- Accepted after revision November 3, 2002.
- Copyright © American Society of Neuroradiology