Abstract
Summary: Subarachnoid hemorrhage from dissecting vertebral artery aneurysms is a well-known phenomenon. With the advent of navigable intracranial stents, these aneurysms are now amenable to endovascular stent placement. However, immediate aneurysm obliteration is often not accomplished, and current covered stents have poor short-term patency rates. We report a series of three patients with intradural dissecting vertebral artery aneurysms treated with a novel endovascular stent-within-a-stent construct using currently available stent technology.
Intradural dissecting vertebral artery (VA) aneurysms represent approximately 3% of all intracranial aneurysms (1, 2), and subarachnoid hemorrhage (SAH) from dissecting VA aneurysms is a well-known phenomenon (3–5). The prompt recognition and treatment of these aneurysms is of utmost importance owing to their high rate of rebleeding in the unsecured state (2–5). Although the most common presentation of these aneurysms is SAH, these aneurysms have been found incidentally in patients who have undergone MR imaging or MR angiography (MRA) for other reasons.
Because of the lack of a clippable neck or a favorable neck-to-sac ratio, dissecting VA aneurysms are often difficult to treat with either microsurgical or endovascular techniques. Proximal ligation or occlusion can sometimes be associated with growth and rebleeding of the aneurysm secondary to reflux across the vertebral confluens (5).
With the advent of navigable intracranial stents, VA aneurysms are now amenable to endovascular stent placement. The stent can be used to exclude the aneurysm from the circulation and preserve the parent artery (6–14). However, immediate aneurysm obliteration is often not accomplished (7, 9), and the current technology of covered stents have poor short-term patency rates (11). Some authors advocate using a stent as a buttress for the implantation of Guglielmi detachable coils or similar embolic devices or agents, but this technique may pose considerable difficulties for the intraprocedural fluoroscopic visualization of coil protrusions (1, 9, 14, 15).
We report a series of three patients with intradural dissecting VA aneurysms treated with a novel endovascular stent-within-a-stent construct by using the currently available stent technology.
Patients and Technique
The patients were taken to the angiography suite after consent was obtained from their family. The procedure was performed with conscious sedation (Versed; Roche Laboratories, Nutley, NJ), which was provided by members of the anesthesiology team. The decision to intubate or not to intubate the patient was left to the team. Systemic intravenous heparin 1000 U/h was used throughout the entire procedure. By using a right common femoral artery approach and 6F Burke coaxial catheters and with guidewire manipulation, the VA was selectively cannulated. Contrast material was injected. Once the aneurysm was located, a Wisdom guidewire (0.014-inch inner diameter, 300-cm length) was advanced beyond the aneurysm. The tip of the wire was depicted within the basilar artery and smoothly advanced. Next, a Velocity balloon-mounted stent was advanced over the wire. The stent was deployed and expanded, and the balloon was then deflated. Angiography was performed to check the patency of the aneurysm. A second, slightly longer stent was next deployed within the first stent to minimize the size of perforations leading to the aneurysm. Run angiography revealed patency of the posterior inferior cerebellar artery. The catheters were removed, and the sheath was left in the groin. The patient was moved to the neurosurgery intensive care unit for monitoring and received heparin 1000 U/h for the next 24 hours. The goal partial thromboplastin time was between 40 and 50. After 24 hours, the heparin was discontinued, and the sheath was subsequently removed.
Case Report 1
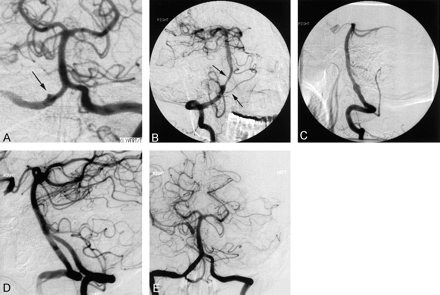
The patient was a 46-year-old right-handed woman who was referred to our facility for further evaluation of a Hunt-Hess grade II SAH. At the referring institution, a cerebral angiogram was reportedly negative for aneurysm or vascular malformations (Fig 1A). A diagnostic angiogram obtained at our institution demonstrated the presence of a 4-mm dissecting aneurysm of the right VA distal to the PICA origin (Fig 1B). Narrowing of more than 50% was present in the right distal vertebrobasilar junction; this finding was indicative of vasospasm.
Case 1. Angiograms in a 46-year-old right-handed woman with an SAH of Hunt-Hess grade II.
A, Left VA injection image obtained at an outside institution. The right VA aneurysm (arrow) was missed because of the failure to perform an ipsilateral injection.
B, Right VA ejection image (anteroposterior [AP] projection) shows dissection and pseudoaneurysm at the origin of the right PICA (bottom arrow). Top arrow indicates vasospasm in the distal VA.
C, Right VA injection image (lateral oblique projection) shows a residual fusiform aneurysm. The area is protected by a stent, and the PICA is patent.
D and E, Right VA injection images (lateral [D and E] projections) obtained 6 months after the stent-within-stent procedure shows complete healing of the aneurysm, with patency of the PICA and restoration of the normal caliber of the vessel.
Endovascular treatment of the right VA dissecting pseudoaneurysm was performed by using an AVE 3 × 18-mm balloon-mounted GFX coronary stent (Advanced Vascular Engineering, Santa Rosa, CA). After systemic heparin was administered, the stent was deployed with 8-atm inflation of the balloon distal to the origin of the PICA within the right vertebrobasilar artery in an attempt to exclude the aneurysm. Post-stenting selective right vertebral images revealed complete normal dilatation of the dissected portion of the VA, but residual filling of the aneurysm was present outside the stent. The stent failed to immediately exclude the aneurysm or cause its thrombosis. We believed that neointimal endothelialization of the stent would develop over time and obliterate the aneurysm.
The patient was readmitted 2 months later for repeat angiography. The right VA injection image revealed good placement and patency of the stent, but the aneurysm still filled (Fig 1C). A second AVE 3 × 18-mm balloon-mounted GFX noncovered coronary stent (Advanced Vascular Engineering) was deployed within the first stent to form a telescoping or stent-within-a-stent construct by using a technique similar to that described previously. This procedure resulted in immediate exclusion and thrombosis of the aneurysm with preservation of the parent artery. Follow-up angiography performed approximately 3 months later showed no residual aneurysm, dissection, or stenosis (Figs 1D and 1E). The PICA origin was patent. The patient remained asymptomatic at 96 months after the stent-within-stent procedure.
Case Report 2
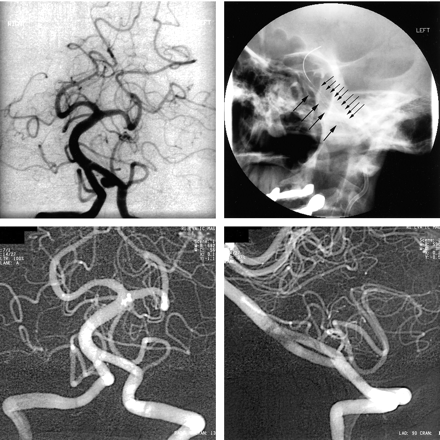
The patient was a 54-year-old man with a history of hypertension who presented to the emergency department with a sudden, severe occipital headache. Head CT revealed an SAH tracking into the ventricles. Clinically, the Hunt-Hess grade was II, and radiographically, the Fisher grade was IV. The patient underwent four-vessel cerebral angiography, and no aneurysms were found. He was admitted to the neurosurgical intensive care unit, he received dilantin and mannitol, and underwent daily transcranial Doppler examination. A repeat angiogram was obtained 9 days later and revealed a 10-mm dissecting left VA aneurysm involving the distal VA just proximal to the PICA origin (Fig 2A). The patient was discharged home and underwent follow-up angiography 3 months later. The images revealed an increase in the size (15 × 12 mm) of the left dissecting VA aneurysm below the origin of the PICA. A Velocity balloon-mounted stent was advanced over the guidewire and deployed. Angiography revealed patency of the aneurysm. A second slightly longer stent was then deployed within the first stent to minimize the size of the perforations leading to the aneurysm. Again, angiography revealed complete opacification of the aneurysm. The patient was then taken to the neurosurgical intensive care unit for monitoring. He was discharged home 3 days later in good neurologic condition. Three months later, the patient underwent follow-up cerebral angiography, which revealed residual opacification of the previously described pseudoaneurysm involving the intradural portion of the left VA (Fig 2B). A significant reduction in the size of the aneurysm was noted. There was no change in the position of the stents. Additional follow-up angiograms were obtained approximately 14 months later; these revealed complete obliteration of the left VA aneurysm, with preservation of the flow in PICA on that side (Fig 2C and D). The patient continued to be neurologically intact.
Angiograms in a 54-year-old man with a history of hypertension and a sudden, severe occipital headache. He had SAH tracking into the ventricles (Hunt-Hess grade II, Fisher grade IV).
A, Left VA injection image (oblique projection) demonstrates a fusiform irregular dissecting aneurysm at the origin of the left PICA.
B, Left VA injection image obtained 6 months after the stent-within-a-stent procedure demonstrates partial resolution of the aneurysm with patency of the PICA.
C (lateral) and D (oblique), Left VA injections images obtained 18 months after the stent-within-a-stent procedure demonstrate complete healing of the aneurysm with restoration of the normal lumen. The stent is across from the origin of the patent PICA.
Case Report 3
The patient was a 52-year-old woman who was referred to our clinic for evaluation of a left VA aneurysm. Her history dated back 2 years before she came to our clinic. She was experiencing episodes of occipital headaches and neck pain associated with vertigo and dizziness. The patient underwent head CT, which showed negative results, followed by MR imaging and MRA, which revealed a fusiform aneurysm of the left VA. Subsequently, she was referred to our clinic for further evaluation. Clinically, she was neurologically intact. Cerebral angiography was performed and demonstrated an 8-mm-long wide-necked aneurysm arising from the anteromedial wall of the distal left VA after the origin of PICA (Fig 3A). The patient agreed to the endovascular procedure, which was performed 2 months later. A Velocity stent (4 × 23 mm) was deployed, and angiography revealed patency of the left PICA, with mild stasis of the contrast material within the aneurysmal sac and slightly delayed filling of the aneurysmal sac. A second Velocity stent (4 × 18 mm) was placed within the first stent. Again, angiography revealed patency of PICA and further slowing of the filling of the aneurysmal sac and further stasis within the aneurysm (Fig 3B). The residual opacification of the aneurysm was likely to have been due to the intraprocedural systemic administration of heparin. The patient tolerated the procedure well and was admitted to the neurosurgical intensive care unit for overnight monitoring and systemic heparin treatment. Six months later, follow-up cerebral angiography revealed healing of the left VA aneurysm with no flow into the aneurysmal sac, as well as patency of PICA and right VA in a retrograde fashion (Fig 3C and D).
Angiograms in a 52-year-old woman who was referred for evaluation of a left VA aneurysm. She was experiencing episodes of occipital headaches and neck pain associated with vertigo and dizziness.
A, Left VA injection image (AP view) demonstrates a left VA intracranial aneurysm distal to the PICA origin.
B, Left VA injection image demonstrates the stent-within-a-stent construct created with two Velocity stents in the left VA.
C (AP view) and D (lateral view), Left VA injection images (AP and lateral views) demonstrate patency of the PICA and complete healing of the aneurysm, with restoration of the normal lumen of the left VA.
Discussion
Since the introduction of intravascular stent placement by Dotter and Judkins in 1964, stent technology has evolved to include the treatment of some intracranial aneurysms (14). Endovascular stent placement with the current technology of noncovered navigable stents may provide an endoluminal matrix for endothelial growth and remodeling of these vessels harboring aneurysms (6–14). The regional effect on the arterial wall with stent placement leads to neointimal formation through transient and regional proliferation and migration of smooth muscle cells mixed with various degrees of connective tissue matrix (16). In experimental studies, this fibrocellular neointima formation generally occurs after 2 weeks (16). Clinical and experimental evidence also demonstrate that intravascular stent placement in aneurysms can alter the inflow and vortex flow within aneurysms, depending on certain hemodynamic parameters, such as the velocity and direction of flow, the location of the aneurysm, the neck diameter, and the particular characteristics intrinsic to the stent. These hemodynamic changes induce the formation of a new flow conduit via the stent that can promote aneurysmal thrombosis. Although convincing experimental evidence suggests that stent placement across an aneurysm neck may, by itself, promote intraluminal thrombosis, the role of this phenomenon in clinical practice may be limited by the high porosity of the currently available stents (9, 14, 17, 18). However, secondary to the high rate of rebleeding and the associated mortality of dissecting aneurysms of the VA, aneurysm exclusion or obliteration is desired.
By deploying a telescoping stent or a stent within a stent across the ostium of an aneurysm, the operator can decrease the porosity of the stent construct. This method can further alter the inflow within the aneurysm, promoting stasis and immediate thrombosis and safely allowing subsequent neointimal endothelial formation. The concept of the placement of a stent within a stent has been adopted largely from isolated case reports in the cardiology literature (19). Fessler et al (20) and Malek et al (21) have both reported the deployment of tandem stents in the intracranial circulation, a rescue stent to treat a dissection distal to the initial stent. However, this is the first case series presenting the placement of a stent within a stent to decrease the porosity of the construct to treat intracranial aneurysms, to our knowledge. In essence, this simple modification of a known endovascular approach attempts to create a pseudocovered stent to exclude the aneurysm from its parent vessel with the currently available stent technology. It avoids the difficulties of the fluoroscopic determination of the position of the coil with stent-coil approaches and the need for the simultaneous use of two microcatheters, a larger guide catheter, and temporary parent-vessel occlusion with balloon-remodeling techniques. Chiaradio et al (22) recently reported the treatment of a dissecting VA aneurysm with an intravascular Jostent graft stent (Jomed, Conroe, TX). The Jostent graft stent is essentially a covered stent, a synthetic graft sandwiched between two stents. Higher-profile devices are more difficult to navigate around the tortuous intracranial circulation, and their use incurs a risk of parent-artery occlusion or reduction of flow sufficient to promote thrombosis.
The AVE Microstent (Arterial Vascular Engineering) is a balloon-expandable device made of 0.008-inch stainless steel wire that is bent in a sinusoidal fashion to form a 3-mm segment. The stent consists of several elements that are laser fused at selected end points. This design purportedly offers greater flexibility and intracranial navigability. The metal surface area in the expanded form is about 14.5% for a stent of 3.5-mm diameter (23).
Although enthusiasm for endovascular stent placement techniques in the treatment of cerebrovascular disease is increasing, this method has several obvious limitations in the treatment of all aneurysms. Most intracranial aneurysms occur at vessel bifurcations and in close association with small perforating branches. Placement of a stent across these bifurcations and the ostia of small perforating branches risks vessel occlusion. With specific reference to dissecting VA aneurysms, this treatment technique may not be an ideal option for aneurysms involving the PICA origin because of the possibility of occluding the PICA territory with the stent. In addition, catheter manipulation and stent placement in the distal vertebral and vertebrobasilar junction risks further dissection, occlusion, or perforation. Experimental evidence in dogs suggests that small, lateral carotid branches that approximate intracranial perforating vessels relative to their diameter and angle of origin remain patent if less than 50% of the ostial diameter is covered by the struts of the stent (9, 17, 24). Despite this evidence, current indications for stent placement in aneurysms are limited to only large lateral pseudoaneurysms near the cranial base and to aneurysms in the intrapetrous and distal intracavernous carotid, the VAs, and along the basilar trunk (14).
The patency rate of stents in the intracranial circulation is unknown, but it most likely decreases over time. The risk of acute stent thrombosis increases, particularly as stent diameters decrease to less than 4 mm (24). This has been shown in the coronary arteries, less so in the intracranial vessels. In small-caliber vessels, an important cause of severe stenosis or subacute parent-vessel occlusion is intimal hyperplasia, a process that can occur as early as 2 weeks to 6 months after stent deployment (7, 14, 16, 25). Stent porosity or the ratio of metal to tissue is another factor associated with neointimal growth. As the porosity decreases or as the stent surface area increases with the use of a telescoping technique, the risk of subacute thrombosis from excessive neointimal growth increases (17, 18). The current limitations will inevitably be mitigated by improvements in stent technology, such as the development of more flexible alloys, smaller introducer catheters, biodegradable nonparticulating material with antithrombotic coatings, covered stents with genetically engineered endothelial cells capable of secreting large amounts of tissue plasminogen activator, stents covered with autologous vein grafts, and custom-designed stents to account for perforating vessels. As stent technology evolves, so too will its applications.
Conclusion
This report presents a case series of three patients with limited follow-up ranging from 6 to 17 months. Prospective studies of comparing this technique with more traditional approaches are needed. However, these cases do illustrate a simple modification of a known method for the treatment of intradural dissecting VA pseudoaneurysms without sacrifice of the parent vessel.
References
- Received December 23, 2002.
- Accepted after revision April 30, 2003.
- Copyright © American Society of Neuroradiology