Abstract
Summary: We present two patients with spinal epidural arteriovenous fistulas involving the vertebral body recruiting dural and osseous branches as feeders. The fistulas, forming a round venous sac into which the multiple arterial feeders converge, were located near the round bony defect of the vertebral body, suggesting the osseous component of this vascular lesion. Transvenous coil embolization of the round venous sac results in near-total obliteration of the lesion, leading to symptomatic improvement.
The limited number of case reports reveals that spinal epidural arteriovenous fistula (AVF) is not a well-defined vascular abnormality in the spine (1–5). Epidural and paraspinal AVF is regarded as the same term, to indicate a high-flow vascular lesion outside the dura or spinal canal (1). They usually have high flow that necessitates transarterial embolization with a balloon or coils to occlude the fistula (2–6).
We present unique features of spinal osseous epidural AVF recruiting multiple arterial feeders into a dilated round venous sac near the vertebral body that is the embolization target via the venous route.
Case Reports
Case 1
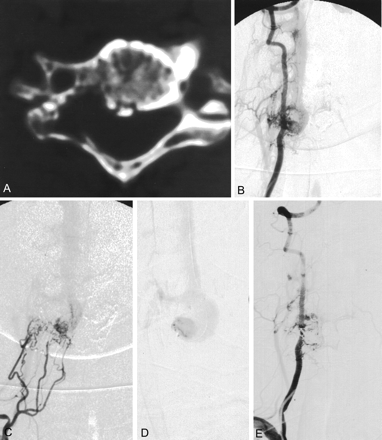
A 50-year-old female patient was referred to the neurosurgical department of our institution for a known cervical arteriovenous malformation. She had experienced intractable cervical neck pain for 3 months. On admission, an audible bruit was detected in the right side of her neck. Findings at neurologic examination were normal. There were no symptoms related to spinal cord compression except for severe radicular pain in her right side. MR imaging performed at another hospital revealed markedly dilated vessels in the epidural area. The spinal cord was compressed by a dilated vessel pouch at the level of the C5 vertebral body. There was a bony defect in the right side of the C5 body and pedicle with loss of cortical margin (Fig 1A). Cervical angiography revealed multiple feeders of fistula converging into the right side of the C5 vertebral body (Fig 1B and C). A round, dilated venous sac arose from the multiple feeders of the fistula and drained into the right jugular vein through the epidural and paravertebral venous plexus. Although the symptom was only a radicular pain, cord compression and annoying bruit warranted further treatment. After a full discussion of surgical and endovascular treatment options with neurosurgeons, we decided to attempt embolization by way of venous approach. Using a transfemoral approach, a 5F catheter was placed into right internal jugular vein and a microcatheter was coaxially introduced through the catheter into the epidural venous space at the C5 level; however, the microcatheter could not be directly advanced into the venous sac at the C5 level because of an occlusion of the venous outflow below the lesion. Therefore, a microcatheter was navigated up to the C1 level through the perivertebral venous plexus, into the epidural vein, and down into the venous sac of the lesion (Fig 1D). Coils were packed into the round venous sac. Because of fistula flow and multiple curves of the microcatheter, rendering coil placement incompletely controllable, packing of the coil was not complete. Nondetachable platinum coils were used to pack the round venous sac because Guglielmi detachable coils (GDCs) were not available at that time. A final angiogram revealed marked reduction of the fistula flow, although there was a faint residual shunt. Her symptoms and bruit disappeared following the procedure. Four-month follow-up angiography (Fig 1E) showed a small amount of residual fistula that did not require further management, because her symptoms had completely resolved. She is doing well, and no recurrent symptoms have been reported during 8 years of follow-up.
Patient 1, a 50-year-old female patient presented with severe radicular pain and bruit in the right cervical neck. Bone window setting of axial CT scan (A) reveals a bony defect with loss of cortical margin in the right side of the C5 body and pedicle corresponding to the area of the dilated venous sac. Anterioposterior views (B and C) of a right vertebral and costocervical arteriogram show multiple fine feeders of fistula draining into a dilated venous sac and cephalad epidural vein. Selective venogram (D) through the microcatheter was obtained in the dilated venous sac. Vertebral angiogram (E) obtained 4 months later reveals reduction of the fistula with a small residual shunt. The pain and bruit disappeared.
Case 2
A 21-year-old female patient was admitted because of leg weakness and voiding difficulty. Her symptoms had progressed since she had mild back pain while walking 1 month earlier. She had no history of trauma. On admission, physical examination revealed grade IV motor power of both legs. Sensory function was intact and symmetric. Deep tendon reflexes were slightly increased on the left side. The Barbinski sign was positive on both sides.
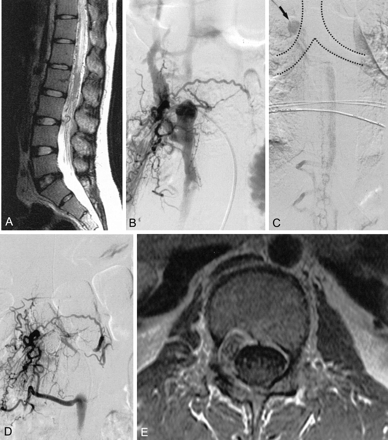
Lumbar MR imaging showed that her spinal cord was swollen and abnormally dilated vessels were noted around the spinal cord (Fig 2A). A dilated venous sac within the L2 body and pedicle were present. Spinal angiography showed multiple small feeders from L2 arteries as well as the right L1- and right L3-level arteries (Fig 2B). Each of the multiple small arterial feeders converged into a round venous sac. Draining into the epidural vein directly, the round venous sac was connected to the epidural vein downward as well as regurgitated into the medullary vein during delayed phase. Because occlusion of the upward epidural venous drainage existed, venous drainage detoured downward via the paravertebral venous plexus, into the epidural vein at the L1 body, and then into the superior vena cava (Fig 2C). The venous outflow tract through the neural foramen level was narrowed. With occlusion of the upward epidural venous flow, the venous pouch formed a rather round sac recruiting multiple small arterial feeders. The microcatheter could not access these arterial feeders of bony and dural branches to the fistula level for embolization.
A 21-year-old female patient presented with both lower leg weakness and voiding difficulty. Sagittal T2-weighted MR image (A) shows the high signal intensity of the diffusely swollen spinal cord. Note the tortuous vessels around the cord surface. Arterial phase of the L2 lumbar artery (B) shows multiple fine feeders of fistula draining into the dilated epidural venous sac. Venous phase (C) of the upper level shows medullary venous engorgement and venous drainage through the hemiazygos vein into the opening site of the azygos vein to the superior vena cava (arrow). Final angiogram (D) after GDC embolization shows complete obliteration of the fistula. Sagittal T2-weighted image (not shown) obtained 6 months later shows the normalized contour and signal intensity of the spinal cord. Contrast-enhanced axial T1-weighted image (E) reveals low signal intensity of the packed coils within the bony defect of the L2 body and pedicle maintaining dural integrity at the site of the dilated venous sac. The patient’s neurologic deficit had disappeared completely.
After discussion with a neurosurgeon and neuroradiologist, an endovascular approach was preferred over surgery to obliterate the fistula. Catheter selection of the main venous channel was not possible through the inferior vena cava via the transfemoral venous route. At the second session, the right internal jugular venous route was punctured with introduction of a 5F sheath. After a 4F hydrophylic catheter was placed in the hemiazygos vein through a 5F sheath placed into, a microcatheter was introduced into the epidural vein. Because of the occlusion of the epidural vein above the lesion, the microcatheter navigated outside of the spinal canal and entered the epidural vein again and finally reached the round venous sac lesion. GDCs were used to pack the lesion. The final angiogram revealed almost complete obliteration of the fistula (Fig 2D). The patient’s symptoms gradually improved and had disappeared completely by the 6-month follow-up examination. Follow-up MR imaging revealed a normalized shape and signal intensity of the spinal cord, leaving the coils mainly within the bony defect of vertebral body and pedicle (Fig 2E).
Discussion
Three major distinctions in spinal vascular malformations and fistulas are made according to the anatomic spaces: 1) subpial and spinal cord arteriovenous malformations, 2) dural arteriovenous shunts, and (3) extradural and paraspinal arteriovenous AVF (7, 8).
Although the actual incidence of spinal dural AVF is not accurately reported, dural AVF is the second most common spinal vascular abnormality next to the intradural arteriovenous malformation, comprising about 30% of all spinal arteriovenous malformations (8, 9). Spinal dural AVF is now regarded as an acquired vascular lesion preceded by venous thrombosis since Manelfe et al (10) described normally existing glomerulus-like arteriovenous connections predominantly in the thoracolumbar region and located between the two layers of dura mater and fed by dural arteries. On the other hand, paraspinal AVF is a rare spinal vascular anomaly known to be induced by congenital or post-traumatic causes (3, 4).
Because epidural AVF has been reported to be a rare category of spinal vascular anomaly, its description has not been definitive and has even been considered to be a variant of dural AVF or paraspinal AVF (1, 9). An epidural AVF reported by Pirouzmand et al (6) showed similar arteriographic findings of dural AVF except for the presence of intradural reflux via the epidural venous plexus far from the primary fistula site in the epidural space and regarded as a variant of spinal dural AVF. The lesion was a fistula from the lateral sacral artery at the S1 level to the epidural venous plexus and reflux to the contralateral S1 medullary vein in a 72-year-old man. The presence of intradural reflux via the epidural venous plexus, far from the primary fistula site in the epidural space, was the cause of progressive paraparesis and was regarded as an unusual manifestation of dural AVF. Epidural AVF in the cervical spine reported by Asai et al (2) had multiple feeders and lesions from C6 to T2. There was, however, no bony lesion or round fistula sac into which feeders converged as in our cases. Among the 14 pediatric spinal arteriovenous malformations reported by Emery et al (5), three cases were epidural. Two of them were infantile high-flow epidural fistulas, and the third was demonstrated on the figures showing low-flow lesions involving the T11 and T12 vertebral bodies. Although a round fistulous nidus was not demonstrated on the figures showing the lesion, it may have been in the same category as ours.
The spinal osseous dural AVF lesion is located in epidural space involving a bone at the area of the dilated venous sac to which all the feeders are converging to the margin. The flow to the sac itself is not as high as that seen with paraspinal AVFs. Therefore, it needs to be categorized differently from other paraspinal spinal AVFs with very high-flow fistulas. To the best of our knowledge, such a disease concept has yet to be described in the literature; we categorized this unique spinal vascular lesion as a spinal osseous epidural AVF because of its characteristic angiographic findings and the effective way of managing it through the venous approach. There are multiple small arterial feeders of segmental arteries converging into a single round venous sac that we call a fistula nidus, because the round venous sac recruits multiple arterial feeders unlike other draining veins. The fistula nidus is located at the site of the bony defect. Because the location of the lesion is epidural, the feeders usually come from the bony and dural branches of the intersegmental arteries. The venous phase reveals the unique features of the fistula nidus connecting to and draining into the epidural vein. Although the arterial flow of the lesion did not seem to be high at the time of diagnosis, venous obstruction as shown in the high-flow angiopathy at the course of the venous drainage occurred at the side of the heart. Therefore, a round, fistula nidus is directly connected to the epidural vein with centrifugal direction from the heart and then drains into the paraspinal venous plexus. When there is disturbance or steno-occlusion at the venous outlet, intradural regurgitation can lead to spinal cord swelling and edema as in our second patient.
The clinical manifestation of spinal osseous epidural AVF seems to be similar to paraspinal or dural AVF. Although we treated only two patients, we presume that patients with spinal osseous epidural AVF usually have progressive neurologic deficit. The symptoms may start as a radicular pain that necessitates MR imaging to find any intervertebral disk herniation, as in our patient 1. Cord symptoms seems to be a late manifestation caused by cord compression due to the dilated venous sac or transmedullary venous congestion that leads to venous stasis in the spinal cord, as in our patient 2.
We believe that obliteration of a round venous sac facing the multiple arterial feeders through venous approach seems to be the treatment of choice for this epidural AVF, although arterial embolization or surgical resection may be possible. Thorough analysis of the venous anatomy is mandatory before selecting the venous route. A jugular venous approach is necessary to find a smoother approach angle and route as in our second patient in whom the venous route began at the right internal jugular vein and finally led into the epidurally dilated venous sac through the superior vena cava, azygos vein, hemiazygos vein, epidural vein, paraspinal venous plexus, and once again the epidural vein in a retrograde fashion. Once the microcatheter arrives at the paravertebral venous plexus, a detour through the opposite direction against the opposite flow of the epidural vein is an important technical point in our patients to allow entry into the fistula nidus, although such a detour makes a venous approach difficult.
Because incomplete coil packing may result in incomplete obliteration of the fistula, dense packing of the coil at the venous sac facing the multiple arterial feeders is critical for the cure of the lesion. After treatment of our first patient, we decided to perform the same approach with GDCs in our second patient. We thereby obtained better control of the coil placement leading to the definite cure of the disease. When nondetachable coils are used, inappropriate coil position and even coil migration may occur, as in our first patient, even though the patient’s symptoms resolved.
In our cases, the bone defect in the dilated round venous fistula sac seems to be different from bony erosion when there was a high-flow fistula with a dilated venous pouch as in a pial AVF with high flow as noted in Rendu-Osler-Weber disease causing a dilated (giant) venous ectagia (11, 12). This venous ectasia compresses the spinal cord and erodes the bony canal within the spinal canal; however, it does not receive the arterial supply to the venous sac, although the lesion is located in the extradural space. Even though we cannot demonstrate the pathologic relationship of this disease category, we presumed that this fistula was located in epidural space near the vertebral bony margin recruiting the multiple feeders of dura and bone based on the anatomic assessment from the angiographic findings and result of the venous embolization of the lesion.
Conclusion
Spinal osseous epidural AVF involving the vertebral body and recruiting the dural and osseous branches as feeders reveals a fistular nidus (ie, a round venous sac) into which the multiple small arterial feeders converge. This corresponds to a round bony defect of the vertebral body, the osseous component of this vascular lesion. Transvenous coil embolization of the round fistular nidus can result in complete obliteration of the multiple arterial feeders of the fistula encircling the round fistular nidus and subsequently lead to symptomatic improvement.
Acknowledgments
We thank Bonie Hami, Department of Radiology, University Hospitals of Cleveland, Cleveland, Ohio, for editorial assistance in manuscript preparation.
Footnotes
Supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (Project No. 03-PJ1-PJ1-CH06-0001).
References
- Received December 24, 2002.
- Accepted after revision April 13, 2003.
- Copyright © American Society of Neuroradiology