Abstract
Summary: We report a case of mechanical thrombectomy in which a new device, the Retriever, was used for acute cerebral ischemia in the setting of extensive occlusion of the left internal carotid and middle cerebral arteries. Excellent radiographic and clinical results were obtained. The Retriever is currently approved and available for foreign body extraction and for intracranial thrombectomy when used as part of the Mechanical Embolus Removal in Cerebral Ischemia, or MERCI, clinical trial. This device was able to retrieve and remove clots efficiently from the intracranial and extracranial circulation, offering a new therapeutic alternative in the treatment of acute cerebral ischemic disease.
Several endovascular mechanical techniques for clot removal or lysis have been developed, and some are currently undergoing clinical trials. These include the use of lasers, angioplasty, ultrasonography, and microsnares, including a device that can physically grasp and remove a thrombus from the cerebral circulation. This report describes the successful mechanical thrombectomy and recanalization of the internal carotid artery (ICA) and middle cerebral artery (MCA) by using the Retriever device (Concentric Medical, Mountain View, CA) in a patient who presented during the acute phase of ischemic stroke.
Case Report
A 54-year-old white male electrical contractor presented to the emergency department approximately 2 hours after the onset of right hemiparesis and aphasia. His wife reported that he had experienced an episode of transient left blindness approximately 4 days before admission. Physical examination revealed attenuated expressive aphasia, flaccid right facial paralysis, and attenuated sensory loss of the right face, arm, and leg. The National Institutes of Health (NIH) stroke scale yielded a value of 24. A CT scan of the head showed a hyperattenuated left MCA without associated ischemic parenchymal changes.
The combination of a high NIH stroke scale and a hyperattenuated MCA sign implies a large clot burden, usually with a poor response to intravenous tissue plasminogen activator (t-PA) (1). A total dose of 4.5 mg of t-PA was administered intravenously (approximately 10% of his total calculated dose of 90 mg) before the patient was assessed by the neuroradiology service. Intra-arterial therapy was recommended, and the intravenous infusion was terminated.
Diagnostic cerebral angiography was performed immediately and revealed a heavily calcified ICA origin plaque with complete occlusion of the left ICA and MCA. A prominent ipsilateral external carotid artery was seen to provide leptomeningeal collateral blood flow to the left MCA territory through branches of the middle meningeal and superficial temporal arteries. Similarly, minimal leptomeningeal collaterals to the left MCA distribution were also provided by peripheral anastomosis of the left anterior and posterior cerebral arteries. The left posterior communicating artery and A1 segment of the left anterior cerebral artery were not visualized and were presumed to be occluded or agenetic. A patent anterior communicating artery (ACA) and peripheral branches of the left ACA were supplied by the right ICA. On the basis of the described functional anatomy, fibrinolysis was not a feasible option owing to absent inflow into the occluded vascular segment. Mechanical thrombectomy was the best therapeutic alternative for emergent neurovascular rescue. The patient’s family was then informed about the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) clinical trial, and appropriate consents were obtained for enrollment in the institutional review board–approved investigation.
Recanalization of the proximal left ICA was initially performed by aspiration thrombectomy by using a 7F guiding catheter. Multiple clot fragments were obtained, and a severe calcified stenosis of the ICA origin was revealed. A 9 × 40 mm Protégé self-expandable stent (ev3 Inc., Plymouth, MN) was deployed to reconstitute the normal proximal ICA vessel caliber. The 7F guiding catheter was exchanged for a 9F concentric balloon occlusion catheter (Concentric Medical, Mountain View, CA), which was positioned within the left ICA at the C2-C3 level. A Rapid Transit microcatheter (Cordis, Miami Lakes, FL) and a Transcend 14 microguidewire (Boston Scientific, Natick, MA) were advanced into the intracranial circulation to catheterize the left MCA. There was absence of flow throughout the intracranial ICA and the entire M1 segment of the MCA, with patency of M2 branches. With the tip of the microcatheter placed at the M1-M2 junction of the MCA, the guidewire was removed and the X6 Retriever device was advanced through the microcatheter and reformed in the M1 segment just proximal to the M1-M2 junction (Fig 1). Once the device was appropriately deployed, the Retriever and the microcatheter were slowly retracted in unison into the balloon catheter under constant suction aspiration. Large fragments of fresh thrombus of an approximate total length of 12 cm were obtained with a single pass of the device (Fig 2).
The Retriever device deployed within the left MCA.
Clot specimen obtained after one pass by using the Retriever embolectomy device.
Angiography demonstrated successful recanalization of the entire left ICA and MCA, with normal antegrade flow into the distal MCA branches. There was no evidence of distal emboli (Fig 3). The patient’s hemiparesis and aphasia began to resolve by the end of the procedure, while the patient was still on the angiography table. NIH stroke scale scores at 2 and 24 hours after the intervention were 9 and 4, respectively. CT conducted 48 hours following intervention demonstrated multiple small focal areas of infarction within the MCA distribution. The hospital course was uneventful, except for mild aspiration pneumonitis, and the patient was discharged home 1 week after admission with very mild expressive aphasia, mild dysarthria, and subtle right facial droop. His motor strength was 4+/5 on the right side, and he was able to return to work the day following discharge.
Digital subtraction angiography following thrombectomy demonstrates complete recanalization of the left internal carotid and MCAs.
Discussion
Ischemic stroke is the third leading cause of death and among the most important causes of disability in the United States. The treatment of acute stroke continues to evolve and improve rapidly, largely because of the successful application of therapeutic strategies for early interventional management (2, 3). The belief that rapid intervention improves the chances of a favorable outcome is the common principle for emergent cerebral revascularization. Large vascular occlusions responded poorly to intravenous t-PA, making the alternative of arterial intervention an excellent therapeutic option (1, 4). Intra-arterial thrombolysis represents a viable alternative but carries the significant risk of cerebral and systemic hemorrhagic complications. When thrombolysis is ineffective or contraindicated, other techniques must be considered to restore flow to territories supplied by large occluded arteries immediately.
Stroke is defined as the sudden onset of an irreversible neurologic deficit. A stroke may be hemorrhagic, due to bleeding into the brain parenchyma or subarachnoid space, or ischemic, due to inadequate blood flow to support the metabolic needs of the brain. Most strokes are ischemic and attributable to cerebrovascular propogation of embolic material, which consists of platelets, red cells, atheromatous debris, or a mixture of these elements. These emboli originate either in the heart or from the surface of the large arteries supplying the brain (5, 6). Whether permanent neurologic injury will occur after an embolic occlusion depends upon three factors: the duration of reduction in blood flow, the degree of the reduction in blood flow, and the intrinsic vulnerability of the affected cells to ischemic injury (7). In the scenario of acute cerebral ischemia, different zones with variable degrees of regional blood flow alteration have been described. A central “ischemic core” with minimal flow, usually <10 mL/100 g brain/min, is destined to progress to infarction. A bordering zone in which the flow and metabolism fluctuate between conditions adverse or favorable for tissue viability is known as the “ischemic penumbra” and typically has blood flow values between 10–15 mL/100 g brain/min (8). On the basis of this information, the concept of emergent treatment of cerebral ischemia and neurovascular rescue has focused upon the preservation and recovery of the ischemic penumbra by limiting the amount of neuronal tissue damage caused by the ischemic process.
Acute ischemic stroke therapy in the emergency department usually involves the use of intravenous t-PA. As a result of two NIH stroke trials that demonstrated a benefit for patients treated within the first 3 hours after stroke symptom onset, the U.S. Food and Drug Administration approved the use of intravenous t-PA in 1996 for the treatment of acute ischemic stroke. Data also support the benefit of intra-arterial thrombolysis in patients with acute stroke treated within 6 hours of symptom onset (9). As a therapeutic intervention, the use of fibrinolytics involves the risk of hemorrhage, either systemic or in the brain. This risk is significant because the blood-brain barrier is disrupted by severe ischemia, predisposing to hemorrhagic transformation of an ischemic infarct (10). Both intravenous and intra-arterial fibrinolysis sometimes fail to achieve recanalization. As a result, mechanical alternatives are available to attempt to achieve rapid revascularization and reperfusion in patients with symptomatic ischemic stroke.
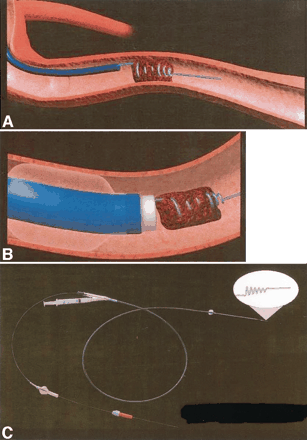
A new mechanical thrombectomy device, the Retriever, is currently being tested in the MERCI clinical trial for acute ischemic stroke within 8 hours of onset. The Retriever consists of a platinum wire with a preshaped, five-loop tapered helix at the distal tip. Two variations are available, the X5 and the slightly stiffer X6, in which the proximal wire diameter is 0.012 inches or 0.014 inches, respectively, and the proximal–distal loop diameters are 2.7 mm and 1.1 mm, respectively. The microcatheter tapers from a proximal diameter of 3F at the hub, to 2.4F at the tip, and has a 0.017-inch inner diameter. The balloon guide consists of a 9F catheter with an inner diameter of 6.4F (0.085 inches), measuring 95 cm in length with a 10 × 10 mm silicone temporary occlusion balloon near its tip. The technical approach includes navigation of the microcatheter over a 0.014-inch guidewire that has passed the occluded segment of a vessel. The wire is removed and angiographic roadmapping is performed to determine vessel diameter, catheter tip location (to ensure a straight segment), and thrombus length. The distal vessel must be greater than or equal to 2 mm to accommodate the two to three distal loops of the device. With the tip of the microcatheter beyond the clotted segment, the Retriever is advanced to allow two to three distal loops of the helix to exit the microcatheter distal to the thrombus. By using angiographic roadmapping, the microcatheter and the Retriever are retracted together a distance of a few millimeters, allowing the distal loops to engage the thrombus. The microcatheter is slowly pulled back over the wire, allowing the proximal loops of the Retriever to be deployed around the thrombus, thereby ensnaring it (Fig 4A). The position of the microcatheter tip is placed approximately 2 mm behind the most proximal loop. Under fluoroscopic guidance, the 9F balloon-guided catheter is inflated sufficiently to occlude flow within the parent ICA vessel. The Retriever is torqued clockwise for a maximum of five revolutions to secure the thrombus within the loops. While the Retriever wire and the microcatheter hub are held together, and suction is applied through the lumen of the balloon guide catheter by using a 20-mL syringe, the microcatheter and the Retriever wire are simultaneously and slowly withdrawn in unison into the guide catheter (Fig 4B and C). The microcatheter and the Retriever are then removed from the guide catheter, which is aspirated to ensure complete evacuation of any clot fragments (11).
Diagrammatic representation of the Retriever embolectomy device.
A, The Retriever wire engaged within the embolus.
B, Balloon guiding catheter and the Retriever wire.
C, Balloon guide catheter and the Retriever wire assembly.
Absolute technical control and careful angiographic evaluation of the occluded and parent vessels is necessary. Significant resistance to clot withdrawal suggests the presence of pre-existing stenosis with atheromatous plaque. In that scenario, the Retriever should be disengaged and removed because of the potential to injure the wall of the occluded vessel if forcefully retracted. Similarly, if the Retriever is unable to negotiate a tortuous bend and starts to unwind, two to three counterclockwise rotations are recommended to reduce the Retriever profile and device tension (11).
Conclusion
Options are available for mechanical thrombectomy in the treatment of acute ischemic stroke. The introduction of a new device, the Retriever, that is able to remove emboli from the intracranial and extracranial cerebral circulation provides a tool for rapid revascularization of ischemic brain tissue with the potential for excellent clinical outcomes. In our case, complete removal of ICA and MCA thrombus was achieved in only a few minutes with a single pass of the Retriever device.
References
- Received September 30, 2003.
- Accepted after revision February 18, 2004.
- Copyright © American Society of Neuroradiology