Abstract
Summary: We present a patient who underwent bone marrow transplantation (BMT) after developing chronic myelocytic leukemia. Four months after BMT, he became comatose and died. MR imaging revealed multifocal brain lesions that were progressive but produced no edema. Postcontrast studies revealed that most of the lesions were nonenhancing. There was only discrete, irregular leptomeningeal enhancement with possible minimal enhancement of the cortex and subcortical white matter. Autopsy showed overwhelming toxoplasmosis encephalitis. This case illustrates that toxoplasmosis lesions may lack obvious contrast enhancement in the brain of the immunocompromised patients, despite severe involvement. Recognition of this unusual MR imaging manifestation of toxoplasmosis should lead to earlier diagnosis and treatment.
Toxoplasmosis encephalitis is an opportunistic infection most commonly seen in AIDS patients. At MR imaging, lesions are multiple, commonly located in the deep central nuclei, posterior fossa or lobar at the gray-white matter junction, with prominent associated mass effect and edema. After gadolinium administration, the lesions typically show intense parenchymal enhancement (1). After bone marrow transplantation (BMT), brain infections occur with a frequency of 2–4%. The spectrum of infections is variable, favoring bacteria, fungi, or viruses (2). Cerebral toxoplasmosis is a rare complication after BMT, with a prevalence of less than 1% in the United States (3). We report the case of a patient who developed fatal cerebral toxoplasmosis characterized by multiple progressive MR imaging lesions that showed a surprising paucity of enhancement and lack of edema on serial studies.
Case Report
Six months after receiving the diagnosis of advanced-stage chronic myelocytic leukemia (CML), a 39 year-old-man received cyclophosphamide 60 mg/kg, antitymocyte globulin and underwent total-body irradiation and allogeneic BMT. Immediately after BMT, he began to receive IV steroids and cyclosporine to prevent graft-versus-host disease. One week after BMT, he developed a neutropenic fever that was treated with broad-spectrum antibiotics, including cephtazidime, acyclovir, and amphotericin-B. IV steroids were discontinued and were not used again. Although he remained neutropenic, the fever subsided and his general condition improved. Approximately 3 months after BMT, he developed headaches. Brain MR imaging was performed (Fig 1). Tacrolimus (FK-506) replaced cyclosporine. The patient developed recurrence of fever, despite continuous antibiotic coverage, and the diagnosis of pneumatosis intestinalis was made. He underwent a right cholectomy with ileostomy. Anaerobic coverage was added. Twenty-four hours after surgery, he developed confusion, lethargy, and inability to follow commands. Neurologic examination was significant for myoclonic jerks involving the right upper and both lower extremities. Laboratory investigations showed a peripheral white blood cell count of 790/L, hemoglobin of 7.1 g/dL, and platelets of 44,000/L. Lumbar puncture revealed CSF protein to be at 137 mg/dL, glucose at 80 mg/dL, and white cell count at 77/dL (100% lymphocytes), and Gram, acid fast bacilli, and India ink stains. Polymerase chain reaction (PCR) results for herpes simplex and toxoplasmosis and cytology findings for malignant cells were negative. Pre- and post-transplant serology findings for toxoplasmosis were previously negative and testing was not repeated during the neurologic illness. Because of the continuous deterioration in the patient’s condition, brain MR imaging was repeated 2 days later, 6 days after the initial MR imaging (Fig 1). During the following week, he became progressively obtunded and comatose. Two additional repeated spinal taps showed increasing protein content in the CSF, but otherwise similar results in comparison to the first spinal tap. PCR for toxoplasma was not repeated in the CSF or serum because of previously negative results. A third MR imaging study, 18 days after the initial one, was performed (Fig 1). Single-voxel MR spectroscopy was performed (Fig 2). Intrathecal chemotherapy was initiated for presumptive metastatic disease, but the patient died 10 days after the onset of neurologic symptoms. Brain autopsy revealed widespread necrotizing toxoplasmosis encephalitis of the cerebrum, cerebellum, basal ganglia, midbrain, and pons, with abundant toxoplasma cysts and trophozoites (Fig 3). No other opportunistic infection, leukemic infiltrate, or lymphoprolipherative disorder was identified. The lungs showed toxoplasmosis pneumonitis, confirmed by specific antibodies.
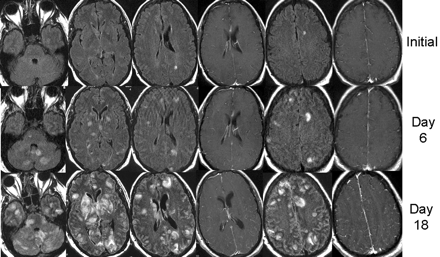
Three serial MR imaging studies obtained during a period of 18 days. Images were obtained by the fluid-attenuated inversion recovery (FLAIR) sequences, with the exception of columns 4 and 6, which were obtained by the postgadolinium T1-weighted sequence. The initial study (top row) revealed a few nonspecific white matter lesions that were thought to be related to cyclosporine toxicity.
The lesions were not seen on noncontrast T1-weighted images or diffusion-weighted images (not shown). After gadolinium administration, lesions were nonenhancing, except for the left parietal-occipital lesion, which shows faint, questionable enhancement. Because of neurologic deterioration, brain MR imaging was repeated (middle row) 6 days after the initial study, revealing worsening lesions in the subcortical white matter, cerebellum, bilateral thalamus, and basal ganglia, with no mass effect or abnormal parenchymal enhancement; there was possibly some increased leptomeningeal enhancement. During the following week, the patient became progressively obtunded and comatose. A third MR imaging study obtained 18 days after the initial study (bottom row) showed continuous progression of lesions in size and distribution, which at this time involved much of the brain. There was a remarkable lack of mass effect and lack of parenchymal enhancement of most lesions; irregular meningeal enhancement with secondary involvement (perhaps by meningeal spread) of the cortex and subcortical areas was suggested. A few lesions were hyperintense on diffusion-weighted images. Apparent diffusion coefficient maps were not obtained to distinguish restricted diffusion versus T2 shine-through (not shown). MR imaging was performed at 1.5 T with the following parameters on each day. Fast spin-echo FLAIR: 5-mm axial sequences with 1-mm section gaps, TR/TE/TI of 10,002/145/2200, matrix of 192 × 256, one signal averaged, and 22-cm field of view. T1-weighted images: 5-mm axial sequences with 1-mm section gaps, TR/TE of 500/20, matrix of 192 × 256, one signal averaged, 22-cm field of view.
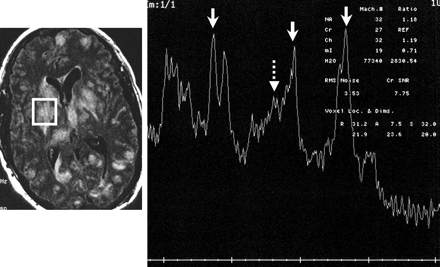
Single- voxel MR spectroscopy (right panel) by using a stimulated-echo acquisition mode with a TR of 1500 ms and TE of 30 ms. The spectral pattern is technically limited and suboptimal (note the poor baseline).
A 2.5 × 2.5 cm voxel of the right putamen lesion was localized based on the FLAIR axial image on day 18 (left panel) at the level of the frontal horns of the lateral ventricle. The spectral pattern shows, from left to right, a moderate increase in choline (solid arrow), a moderate reduction in n-acetyl-aspartate (solid arrow), and a marked lactate peak (solid arrow). There is also a mild prominence of (amino acid) peak (dashed arrow).
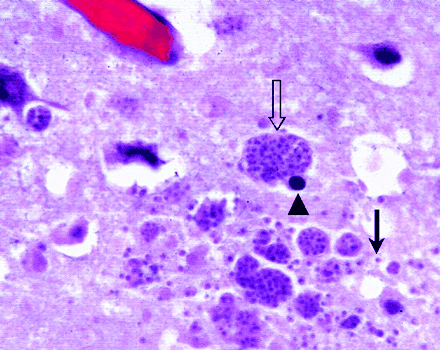
Autopsy findings. Photomicrograph (magnification ×600, hematoxylin and eosin stain) of a histologic section of the cerebrum reveals multiple toxoplasma cysts (open arrow), probable neuronal cells with round, dark nuclei (arrowhead), and additional trophozoites in the extracellular space after the cysts rupture (straight arrow). The findings are consistent with those of toxoplasmosis encephalitis.
Discussion
Our case is an example of a non-AIDS immunocompromised patient with severe multiorgan toxoplasmosis and a fulminant course, rapid deterioration, and death. Despite significant cerebral involvement, the diagnosis was not made during life. The paucity of enhancement and lack of edema were considered unusual for bacterial, parasitic, or fungal infections. Initial MR imaging showing multiple lesions involving the posterior fossa and subcortical white matter raised the possibility of cyclosporine toxicity. Progressive multifocal leukoencephalopathy or lymphoproliferative disorders were considered remote possibilities. The clinical course and initially negative diffusion-weighted findings did not support the diagnosis of stroke.
In the United States, toxoplasma encephalitis is a rare condition in non-AIDS patients, occurring with a frequency of 0.31% of BMT patients. The disease is extremely rare in seronegative patients, especially in syngeneic recipients, but it can complicate an allogeneic BMT, suggesting an acquired infection (4). Immunohistochemistry yields positive results concordant with autopsy in only 65% of cases, whereas PCR for toxoplasmosis DNA is positive in only 75% of cases (5). Our case highlights the limited sensitivity of PCR in diagnosing toxoplasmosis in BMT patients.
Brain MR imaging in AIDS-related cerebral toxoplasmosis usually shows multiple lesions in the deep gray and subcortical white matter–gray matter junction of variable size and enhancement patterns (1, 4, 6–9). Other common locations include the posterior fossa, cortical gray matter, and periventricular white matter. Toxoplasmosis lesions are usually associated with copious surrounding edema and marked mass effect. After contrast administration, intense parenchymal enhancement is nearly ubiquitous, whereas in our case we saw only subtle, irregular meningeal enhancement with possible extension to the cortex and white matter–gray matter junction. Most of the parenchymal lesions, however, were nonenhancing. To the best of our knowledge, brain toxoplasmosis with such a paucity of enhancement has not been reported in patients with AIDS. In toxoplasma encephalitis complicating BMT, a lack of enhancement is occasionally encountered for small nodular lesions or sometimes may be masked by T1 shortening due to hemorrhagic transformation in larger lesions. The percentage of nonenhancing cases is unknown in the United States, but in Europe it has been reported to be as high as 35%. Even when enhancement is lacking, however, the mass effect and edema are usually present (10, 11).
MR spectroscopy was of questionable value in the current case. The study was performed primarily to exclude neoplastic disease, which was not suggested by the MR spectroscopy pattern (9). The literature regarding MR spectroscopy in toxoplasmosis is sparse when referring exclusively to AIDS patients. A decreased n-acetylaspartate, moderately decreased choline, markedly increased lactate and lipid, and absent myoinositol peak may be seen in cerebral toxoplasmosis (12, 13). In our patient, MR spectroscopy showed a similar pattern, with the addition of a possible abnormal amino acid peak. It is not clear whether MR spectroscopy can differentiate toxoplasmosis from other lesions that occur in immunocompromised hosts such as brain lymphoma. The MR spectroscopy findings in our case was technically limited and nonspecific, and the pattern could be seen in a host of inflammatory and infectious diseases (9).
Our case suggests that toxoplasmosis encephalitis may manifest differently in AIDS versus non-AIDS immunocompromised hosts. We speculate that this is due to the immunocompromised state being inherently different in these two conditions. AIDS is characterized by selective CD4 cell loss (related to human immunodeficiency virus), whereas post-BMT patients have more global loss of immune cells because of the effect of immunosuppressive medications. Thus, the inflammatory response may differ between these two groups in the face of an opportunistic infection, and post-BMT patients in particular may not mount a sufficient inflammatory response at the blood-brain barrier to allow passage of gadolinium and vasogenic edema.
Corticosteroids can also blunt the immune response and reduce enhancement associated with inflammatory diseases; however, our patient was not receiving steroids in the 2 months before the onset of the neurologic illness. Therefore, we emphasize that cerebral toxoplasmosis can complicate BMT and may have an unusual MR imaging presentation, different from the expected pattern encountered in AIDS.
Conclusion
Cerebral toxoplasmosis should be considered in BMT patients with an unexplained neurologic deterioration despite a lack of enhancement or mass effect at MR imaging.
References
- Received March 18, 2003.
- Accepted after revision May 6, 2003.
- Copyright © American Society of Neuroradiology