Abstract
BACKGROUND AND PURPOSE: Oxygen has a known paramagnetic effect and increases CSF signal intensity on fluid-attenuated inversion recovery (FLAIR) MR images. The purposes of this study were to investigate the effect of supplemental oxygen on CSF signal intensity and the arterial partial pressure of oxygen and to determine the possible synergistic effect of oxygen and albumin on T1 shortening effect in vitro.
METHODS: Six healthy volunteers underwent FLAIR MR imaging of the brain before and during inhalation of 10 to 15 L/min of 100% oxygen for ≤30 min. The signal intensity was measured in the subarachnoid spaces and various tissues and correlated with estimated arterial partial pressure of oxygen and arterial carbon dioxide pressure. In vitro measurements were also obtained by using two sets of saline-filled tubes with various concentrations of albumin, one of which was exposed to increased oxygen levels. In vitro T1 relaxation times were calculated to assess the possible synergistic effect of oxygen and albumin.
RESULTS: FLAIR images of healthy volunteers showed increased CSF signal intensity within the basal cisterns and sulci along the cerebral convexities. The CSF hyperintensity was observed immediately after the initiation of supplemental oxygen and remained stable during the oxygen administration. There was approximately a 4- to 5.3-fold increase in signal intensity with supplemental oxygen. The phantom experiments showed a T1 shortening effect of oxygen. Albumin significantly altered T1 relaxation time only at high concentrations of albumin.
CONCLUSION: Inhalation of increased levels of oxygen led to readily detectable CSF hyperintensity on FLAIR images of healthy volunteers. No significant synergetic effect of albumin and oxygen was noted.
Fluid-attenuated inversion recovery (FLAIR) imaging sequences have been used widely for MR imaging of the brain and have become an essential part of routine MR imaging of the brain (1, 2). High signal intensity of CSF on FLAIR images is seen in the setting of subarachnoid hemorrhage or meningitis, reflecting the T1 shortening effect of protein in CSF (3–6). CSF hyperintensity has also been reported in patients who underwent MR imaging examinations while under general anesthesia. This CSF hyperintensity on FLAIR images was initially thought to be secondary to inhalation anesthetics or slight increase in protein content in CSF. A study of MR imaging of the brain in pediatric patients reported that CSF hyperintensity on was seen on the FLAIR images of patients who received propofol, which is a highly lipophilic IV administered anesthetic agent (7). These signal intensity changes were not seen on the FLAIR images of patients who received chloral hydrate. Deliganis et al (8) reported that high signal intensity of CSF on FLAIR images of anesthetized patients was caused by the paramagnetic effects of supplemental oxygen. They further found that with in vitro testing, the anesthetics had no notable T1 shortening effects. Although the paramagnetic effect of supplemental oxygen was shown in healthy volunteers, the degree of CSF enhancement was less than that shown for patients under general anesthesia. They suggested that the difference in the degree of CSF hyperintensity could be due to the oxygen delivery method or could be due to abnormality of CSF in the patient group. We hypothesized that if CSF protein concentration was increased in the patients and if a synergistic effect existed between protein and oxygen relaxivity, those factors also might account for the difference in CSF signal intensity on FLAIR images. It is difficult to accurately evaluate such interaction between oxygen and protein in CSF in vivo; thus, the phantom study was conducted to determine the T1 shortening effect of albumin and oxygen in a well-controlled environment.
The aims of this study were to investigate the paramagnetic effect of supplemental oxygen on CSF signal intensity with various oxygen delivery methods, to correlate the effect with estimated arterial partial pressure of oxygen, and to determine the possible synergistic effect of albumin in a phantom study to determine whether albumin potentiates the paramagnetic effect of oxygen.
Methods
This study consisted of three parts: 1) MR imaging of healthy volunteers with supplemental oxygen, 2) oxygen partial pressure measurement with supplemental oxygen, and 3) in vitro study to investigate the synergetic effect of albumin on the paramagnetic effect of oxygen. The study was approved by our institutional review board, and all healthy volunteers provided signed informed consent.
MR Imaging of Healthy Volunteers
Six healthy volunteers (three men and three women; average age, 37 years; age range, 34–40 years) underwent FLAIR MR imaging before and during administration of 15 L/min of 100% of oxygen by mask. Two different types of masks were used: a loose mask and a non-rebreathing sealed mask with a closed reservoir. Three volunteers inhaled oxygen through the loose mask. FLAIR images were obtained 30 min after beginning inhalation of oxygen. The other three volunteers inhaled oxygen through the non-rebreathing sealed mask. After the acquisition of baseline images, FLAIR images were acquired sequentially every 5 min during a 30-min period of oxygen administration.
MR Imaging and Region of Interest Analysis
MR imaging was performed with a 1.5-T system. A fast FLAIR sequence was obtained for each participant. The fast FLAIR sequence consisted the following fixed parameters: 10,000/157 (TR/effective TE); inversion time, 2200 ms; field of view, 22 × 16 cm; matrix, 256 × 192; section thickness, 5 mm.
Quantitative data were obtained by placing a small region of interest in the subarachnoid spaces (prepontine, ambient, and interpeduncular cisterns and sulci of cerebral convex), lateral ventricle, choroid plexus, gray and white matter, and globe (vitreous). Three regions of interest were placed for each anatomic area. The average crude signal intensity was normalized to signal intensity of white matter, because no oxygen effect has been seen in white matter (8). Signal intensity changes were calculated by using the following formula:
(SI of CSF after O2/SI of WM after O2)−(SI of CSF before O2/SI of WM before O2)/(SI of CSF before O2/SI of WM before O2) where SI signifies signal intensity and WM, white matter.
Oxygen Partial Pressure Measurement
To estimate arterial partial pressure of oxygen (PtcO2) and arterial carbon dioxide pressure, six healthy volunteers underwent transcutaneous measurement of partial pressure of oxygen and transcutaneous measurement of partial pressure of carbon dioxide (PtcCO2) outside the MR imaging unit before and during inhalation of 15 L of 100% oxygen by mask. Transcutaneous measurement of partial pressure of oxygen and carbon dioxide were achieved by using a Micro-gas 7650 (Sensor Medics, Yorba Linda, CA), which was not MR compatible. The baseline transcutaneous measurement of partial pressure of oxygen and carbon dioxide were measured while the participants were calmly sitting on a chair before administration of oxygen. Two different types of masks, a loose mask and a non-rebreathing sealed mask, were used. After acquisition of baseline transcutaneous measurement of partial pressure of oxygen and carbon dioxide, repeated measurements were acquired sequentially every 5 min during oxygen administration, for a total of 20 min. With each participant, after acquiring data with the use of one type of mask, measurement was repeated with the use of the second type of mask after transcutaneous measurement of partial pressure of oxygen returned to the baseline.
In Vitro Studies
To investigate the potential synergetic effect of albumin and oxygen, we performed in vitro testing by using two sets of saline-filled glass tubes (15 mL) mixed with nine different concentrations of albumin, raging from 0 to 2500 mg/dL. One set of tubes was equilibrated with 100% oxygen at rate of 0.5 L/min for 3 min. Oxygen was administered through a spinal needle that had been inserted into the depth of the saline-filled tubes. The tubes were vented through a second short needle that had been inserted into the top of the tubes, the tip of needle located just beneath the rubber stopper.
T1 relaxation time in the phantom tubes were measured by using multi-echo, multiple saturation recovery and modified fast inversion recovery sequences with fast spin-echo signal intensity readout (9). Images of the phantom were obtained by using a standard transmit-receive head coil with the following parameters: 4000/15; echo train length, 6; and variable inversion times, 3500, 3000, 2500, 2000, 1500, 1250, 1000, and 500 ms. All images were acquired for a single section with a thickness of 6 mm while keeping receiver and transmitter gains constant. Signal intensities were measured in identical regions of interest placed within each tube. T1 values were obtained from a three-parameter fit of the following equation: 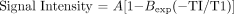
where adjustable parameters A and B absorb contributions of spin attenuation, T2, and actual flip angles (9–11). Exp signifies exponential.
Statistical Analysis
Paired two-tail t test was used to analyze statistical significance of transcutaneous measurement of partial pressure of oxygen and carbon dioxide before and after oxygen inhalation, as well as post-oxygen partial pressure between loose and sealed masks.
Results
Imaging of Healthy Volunteers
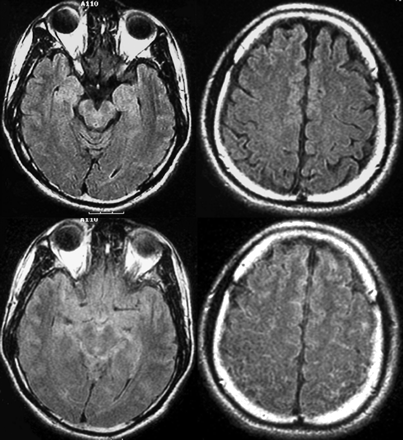
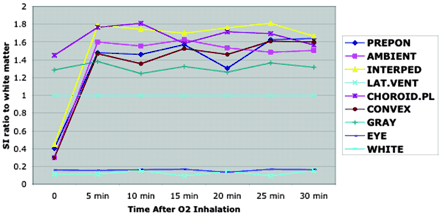
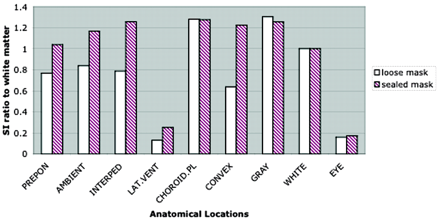
FLAIR images of healthy volunteers with supplemental oxygen clearly showed increased CSF signal intensity in the basal cisterns and sulci along the cerebral convexities (Fig 1). Increased CSF signal intensity was seen most consistently and intensely within the basal cisterns, including the interpeduncular, prepontine, ambient cisterns, and sulci over the convexity. The sequential study that used a sealed mask showed 4.0- to 5.3-fold increases in CSF signal intensity at the basal cisterns and sulci over the convexity (Fig 2) (P < .0001 at 30 min after 02 inhalation). The signal intensity of the choroid plexus, lateral ventricles, gray matter, and vitreous humor remained unchanged. The increase in CSF signal intensity was present by the first observation at 5 min after the initiation of supplemental oxygen and remained stable thereafter. The CSF hyperintensity was more pronounced with the non-rebreathing sealed mask compared with the loose mask (Fig 3) (P < .01). The signal intensity ratio normalized to white matter was approximately 50% higher with the sealed mask than with the loose mask at 30 min after administration of oxygen.
FLAIR images of a healthy volunteer before (upper) and after (lower) inhalation of 100% oxygen with the use of a sealed mask. CSF hyperintensity is clearly visible in the basilar cistern (left) and sulci over the cerebral convexities (right) after oxygen inhalation.
Sequential signal intensity ratio change. Signal intensity of various structures normalized to white matter as a function of time after initiation of supplemental oxygen to volunteers shows that signal intensity of prepontine, ambient, and interpeduncular cisterns and sulci over the convexity increases rapidly after oxygen inhalation. No signal intensity changes are seen in the lateral ventricles, brain parenchyma, vitreous (eye), and choroid plexus. PREPON, prepontine cistern; AMBIENT, ambient cistern; INTERPED, interpeduncular cistern; LAT.VENT, lateral ventricle; CHOROID.PL, choroid plexus; CONVEX, sulci over the convexity; GRAY, gray matter; EYE, vitreous of eye; WHITE, white matter.
Signal intensity to white matter ratio. Measurements of signal intensity ratios of various anatomic structures in healthy volunteers at 30 min with the use of two types of masks show that CSF hyperintensity is significantly higher with the sealed mask compared with the loose mask (P < .01). PREPON, prepontine cistern; AMBIENT, ambient cistern; INTERPED, interpeduncular cistern; LAT.VENT, lateral ventricle; CHOROID.PL, choroid plexus; CONVEX, sulci over the convexity; GRAY, gray matter; EYE, vitreous of eye; WHITE, white matter.
Oxygen Tension Measurements
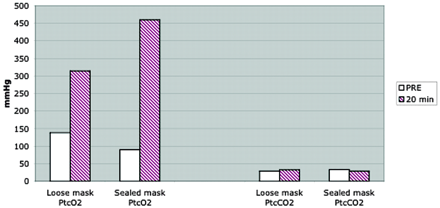
The baseline transcutaneous measurement of partial pressure of oxygen and carbon dioxide of six participants were 114 ± 33 mmHg (average ± SD) and 33 ± 8 mmHg, respectively. Inhalation of 100% of oxygen dramatically increased transcutaneous measurement of partial pressure of oxygen with both masks, which was statistically significant (P < .01 for loose mask and P < .0001 for sealed mask). Mean maximum transcutaneous measurement of partial pressure of oxygen with the non-rebreathing sealed mask was 460 ± 103 mmHg and was significantly higher than that, 312 ± 84 mmHg, with the loose mask (P < .05) (Fig 4). No significant difference in transcutaneous measurement of partial pressure of carbon dioxide was noted between loose mask (33 ± 7 mmHg) and nonrebreathing sealed mask (36 ± 6 mmHg), and no significant changes (P = .96) in transcutaneous measurement of partial pressure of carbon dioxide were noted before or after oxygen inhalation. These results indicate that the oxygen delivery method used in this study of healthy volunteers yielded a 5-fold increase in transcutaneous measurement of partial pressure of oxygen with a sealed mask and 2.3-fold increase in transcutaneous measurement of partial pressure of oxygen with a loose mask (Fig 4).
Transcutaneous oxymetry. Measurement of transcutaneous partial pressure of oxygen (PtcO2) and carbon dioxide (PtcCO2) in six healthy volunteers shows markedly high transcutaneous measurement of partial pressure of oxygen (ranging from 300–500 mmHg) after inhalation of 100% oxygen at 20 min. Transcutaneous measurement of partial pressure of oxygen was substantially higher when a sealed mask was used, compared with a loose mask. Transcutaneous measurement of partial pressure of carbon dioxide remains low, and no significant difference (P = .96) was seen between the loose mask and the sealed mask.
In Vitro Studies
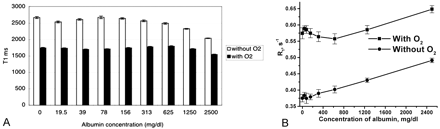
T1 relaxation time measurements in vitro showed marked shortening of T1 relaxation time in a set of tubes exposed to oxygen compared with the set without oxygen exposure. The T1 shortening effect of oxygen was relatively independent of the albumin concentration. Overall, >30% reduction of T1 relaxation time occurred with supplemental oxygen. The lower concentrations of albumin did not notably alter T1 relaxation time, which only measurably shorten with higher concentrations of albumin at 1250 and 2500 mg/dL (Fig 5A). However, if we present the data as a relaxation rate, R1 = 1/T1, a nearly linear plot of R1 as a function of concentration of albumin is noted (Fig 5B). The curve for T1 shortening (increasing T1 relaxivity) with increasing albumin concentration with oxygen was nearly parallel to the curve without oxygen, indicating that there was no substantial synergistic effect of oxygen and albumin.
Lower concentrations of albumin did not significantly alter T1 relaxation time.
A, T1 relaxation time measurement of a phantom with various degrees of albumin concentration shows marked reduction of T1 relaxation time in a set of tubes with oxygen exposure. No notable changes of T1 relaxation time are seen in a set of tubes without oxygen until the albumin concentration reaches 1250 mg/dL.
B, Same data were presented as TR (R1 = 1/T1) with various degree of albumin concentration. These two graphs show nearly linear relaxivity with or without oxygen. The curves of T1 relaxation time with and without oxygen are almost parallel, suggestive of no significant synergistic effect of albumin on T1 shortening of oxygen.
Discussion
Oxygen, with two unpaired electrons, is weakly paramagnetic. This paramagnetic effect of oxygen has been noted in multiple tissues, including myocardium, spleen, and arteries (12), and has been explored as a potential contrast agent for MR ventilation studies of lungs (8, 9) and as a contrast agent in the brain (10) and arterial blood (11). The effect of oxygen on CSF has been less extensively studied.
With the present study, we showed that administration of supplemental oxygen by face mask results in markedly increased signal intensity of CSF on FLAIR images of healthy volunteers. We also showed that exposure to oxygen shortens the T1 relaxation time of saline in vitro, which is the likely mechanism to account for the hyperintensity seen on FLAIR images. The signal intensity of CSF on FLAIR images of the volunteers studied increased by the first imaging interval at 5 min after the initiation of supplemental oxygen and remained stable thereafter. A synergetic effect of increased protein in the CSF and increased oxygen levels was speculated, but in vitro testing revealed that there was no synergetic effect between albumin and oxygen except for very high concentrations of albumin.
Normally, in room air, the arterial oxygen saturation by hemoglobin is close to 100%. The partial pressure of dissolved oxygen in the blood (at sea level) represents a small fraction of the total oxygen concentration (<0.3%). As supplemental oxygen is increased, the concentration of dissolved oxygen in the blood is increased. Dissolved oxygen in the blood will diffuse into tissues according to the oxygen gradient. Because CSF has no oxygen-carrying molecule, such as hemoglobin or myoglobin, the total concentration of oxygen in the CSF will be the dissolved oxygen.
The relationship between CSF oxygen tension and arterial partial pressure of oxygen was examined in a rabbit model in 1979 (12). CSF oxygen tension was linearly related to arterial partial pressure of oxygen, and the diffusion of oxygen from blood at the blood-CSF border was presumed to be the main mechanism of such transfer (12, 13). It has been long thought that the direct transfer of oxygen from serum to CSF took place primarily in the ventricles through the choroid plexus (12–15). As shown in our results, however, an immediate increase in signal intensity was seen in the basal cisterns and sulci over the convexities without notable signal intensity changes within the lateral ventricles or choroid plexus. Our results suggest that diffusion of oxygen from the blood to the CSF takes place along the pia-arachnoid surface of the brain rather than in the choroid plexus.
A few potential explanations exist for this regional appearance of the CSF hyperintensity. First, the basilar cistern is surrounded by major cranial vessels, with abundant pia arachnoid surface areas permitting diffusion of free oxygen to CSF. Increased signal intensity wasn’t seen in the ventricles, which are lined by less vascular ependymal cells. Second, the amount of CSF surrounding the basilar cistern is small, so there will be less dilution of dissolved oxygen in this region than in the lateral ventricles, which contain a substantially larger amount of CSF. Thus, even though diffusion of dissolved oxygen is present at the choroid plexus, the signal intensity of the lateral ventricles may not be significantly increased. In addition, there may also be small effect mediated by the blood-brain barrier. The brain functions within a well-controlled environment in the presence of tight junctions between cerebral endothelial cells that create the blood-brain barrier (16). The blood-brain barrier near the pituitary gland is known to be one of the “leaky” areas of blood-brain barrier, having fenestrations with incomplete tight junctions. This may potentially facilitate the transport of dissolved oxygen into CSF in this region.
CSF hyperintensity in patients under general anesthesia was reported to be much greater than that seen in healthy volunteers or patients under conscious sedation (17). Arterial partial pressure of oxygen of an intubated patient with a fraction of inspired oxygen of 1.0 would theoretically reach 671 mmHg but typically is found to be in the range of 300 to 400 mmHg in anesthetized patients receiving a high fraction of inspired oxygen. Deliganis et al (8) reported that there was no paramagnetic effect of anesthetics to account for CSF signal intensity. A potential explanation for the greater CSF hyperintensity in anesthetized patients is that oxygen delivery via intubation is more efficient than via the sealed face mask or nasal canula. Face mask and nasal canula delivery of supplemental oxygen will result in entraining of some room air, which will limit the fraction of inspired oxygen and be less than that achievable in an intubated patient. When oxygen is administered during general anesthesia by using a breathing circuit containing a reservoir (bag or bellows), arterial partial pressure of oxygen may rapidly increase, leading to a high concentration of dissolved oxygen for a short period of time, which may facilitate diffusion of serum-free oxygen to the CSF more efficiently than with a nasal canula or face mask. A retrospective review of FLAIR MR imaging of the brain in pediatric patients who were under general anesthesia revealed that CSF hyperintensity was more prominent in patients who had a higher fraction of inspired oxygen (>0.60) compared with patients who had a lower fraction of inspired oxygen (<0.60) (18).
Anesthetics may reduce oxygen consumption of the brain, resulting in more dissolved oxygen available in CSF. A comatose patient has approximately 20% to 35% reduction of oxygen consumption of the brain compared with an awake healthy adult (19). However, this effect is probably minimal considering that oxygen consumption of the brain is relatively well controlled to steady state in most persons. If the blood-brain barrier contributes oxygen transfer from serum to CSF, blood flow may affect such transfer, because oxygen diffusion at the blood-brain barrier depends on regional blood flow (20). The investigation of this mechanism requires further studies.
Various FLAIR imaging parameters could affect the T1 shortening effect of oxygen in CSF. Because oxygenated CSF has shorter T1 relaxation time, by properly reducing inversion time, the signal intensity from oxygenated CSF can be nulled (21). At the same time, signal intensity from normal CSF will be elevated in the setting of shortening inversion time. When short relaxation time is used for FLAIR imaging, inversion time to suppress normal CSF is also shortened accordingly. This may result in smaller residual magnetization of oxygenated CSF, thus potentially less sensitive to oxygen effect. In addition, adjustments of FLAIR imaging parameters may affect tissue contrast in the brain (22). With short inversion time, one may expect unwanted suppression of the signal intensity from lesions having long T1 and gray matter (21). Although we did not measure T2 relaxation time in our study, T2 shortening effect of oxygen is expected to be small. Melhem et al (23) investigated the threshold of protein concentration for CSF hyperintensity. They showed that the threshold depended on the effective TE in FLAIR sequence. As the T2 weighting increases, the albumin concentration thresholds decrease (the longer the TE, the lower the albumin concentration yielding hyperintensity on FLAIR sequences). More detailed assessment of the effects of each MR imaging parameter requires mathematical modeling, which is beyond the scope of this article.
In vitro measurements in our study showed that oxygen has a stronger T1 shortening effect than does albumin. Oxygen reduced T1 relaxation time >30% without albumin. Contrary to this, T1 relaxation time seems to decrease significantly only when the concentration of albumin reaches 1250 or 2500 mg. Presenting data in relaxivity rate confirms the standard linear model of relaxivity by albumin.
Taoka et al (24) , in a retrospective review of 300 clinical MR imaging studies, reported CSF hyperintensity on FLAIR MR images without apparent CSF abnormality. They speculated that such “dirty CSF” sign was frequently associated with mass effect, vascular anomaly, and vascular enhancement and speculated that such CSF hyperintensity might have been due to protein leakage or CSF flow phenomenon. Our prospective study in healthy volunteers clearly showed that CSF hyperintensity can occur after only supplemental oxygen administration and is not related to CSF flow phenomenon or protein leakage.
Conclusion
We confirmed that high signal intensity of CSF on FLAIR images is caused by inhalation of oxygen in healthy volunteers. The observation is clinically important to avoid misinterpretation. The T1 shortening effect of oxygen was not significantly enhanced with low concentrations of albumin. Markedly increased CSF signal intensity in patients who are under general anesthesia is mainly due to a more efficient oxygen delivery system leading to greater increased dissolved free oxygen in the CSF.
References
- Received June 17, 2003.
- Accepted after revision July 31, 2003.
- Copyright © American Society of Neuroradiology