Abstract
BACKGROUND AND PURPOSE: Aneurysms with a wide neck constitute a persistent challenge for endovascular therapy with coils. Our purpose was to evaluate the immediate anatomic and clinical results of treating intracranial wide-necked aneurysms by using three-dimensional (3D) coils.
METHODS: During a 2-year period, 160 aneurysms (116 with a neck ≤ 4 mm, group A; 44 with a neck > 4 mm, group B) in 157 patients in eight participating centers were consecutively treated. The procedure consisted first of framing the aneurysm with one or more 3D spherical coils and then filling it with helical coils. Results were evaluated with univariate analysis. Multivariate analysis was used to identify independent predictors of these results.
RESULTS: Angiographic occlusion was complete in 84 (72%) and 30 (68%) aneurysms in groups A and B, respectively. Mean percentage of volumic occlusion in these groups was 30.9% and 29.2%, respectively. Perioperative morbidity and mortality rates were 4%, respectively, in group A and 2%, respectively, in group B. No significant difference between the two groups was observed. However, percentage of volumic occlusion correlated with sac-to-neck ratio less than 1.5 (P = .061) and with sac size (P = .002) except when three or more 3D coils per aneurysm were used (P = .222). The better the percentage of volumic occlusion, the better the degree of angiographic occlusion (P = .004). Percentage of volumic occlusion was an independent predictor of angiographic complete occlusion (P = .001). World Federation of Neurological Surgeons subarachnoid hemorrhage scale grade 5 was an independent predictor of perioperative mortality (P = .043).
CONCLUSION: Three-dimensional coils proved to be useful for improving coil packing and angiographic and volumic occlusion of aneurysms with a neck greater than 4 mm, at the time of treatment, provided the sac-to-neck ratio was 1.5 or greater, and the largest number of 3D coils were first positioned.
Considerable progress in endovascular techniques and the development of new devices such as electrolytically detachable Guglielmi coils (GDC; Target Therapeutics/ Boston Scientific, Fremont, CA) have dramatically changed the therapeutic modalities for managing intracranial aneurysms (1, 2). In large series with coil occlusion, however, wide-necked aneurysms usually correlated with a lower aneurysmal occlusion rate in the period immediately after treatment and a higher recanalization rate than the corresponding rates for small-necked aneurysms (3–9). Use of three-dimensional (3D) GDCs enabled the treatment of intracranial aneurysms with an unfavorable geometry, which would otherwise not be amenable to endovascular treatment without adjunctive techniques (10, 11). Spherical Micrus microcoils (Micrus Corporation, Sunnyvale, CA) are newly designed coils with a high 3D shape memory. Three-dimensional coils may prove useful in improving the quality of spherical support within the aneurysmal sac when one or more successive 3D coils are first positioned and consequently also may improve the efficacy of aneurysmal occlusion and coil packing of embolized aneurysms. The purpose of the present investigation was to evaluate the respective results for wide-necked and small-necked aneurysms as regard the safety and efficacy of endovascular treatment by initial placement of one or more 3D Micrus coils, and to identify any predictors of these results.
Methods
Patient Population
From November 2000 to December 2002, 157 patients (103 women and 54 men) with 160 unselected consecutive intracranial aneurysms were treated with Micrus microcoils in eight interventional neuroradiology centers, after approval by their institutional review boards. Patients were aged 28–83 years (mean, 51 years; median, 49 years). One hundred two patients (65%) had subarachnoid hemorrhage, three (2%) had a mass effect, and 52 (33%) were asymptomatic. One hundred two aneurysms (64%) were ruptured and 58 (36%) were unruptured (Table 1). Patients with ruptured aneurysms were clinically assessed by using the World Federation of Neurologic Surgeons (WFNS) subarachnoid hemorrhage grading scale (12). At admission, WFNS grades were assigned as follows: grade 1 in 34 patients (33%), grade 2 in 14 patients (14%), grade 3 in 14 patients (14%), grade 4 in 26 patients (25%), and grade 5 in 14 patients (14%).
Aneurysms treated with 3D spherical coils in eight interventional neuroradiology centers
Aneurysmal Features
Aneurysm Location.—
One hundred thirty-eight aneurysms (86%) were located in the anterior circulation, and 22 (14%) in the posterior circulation (Table 2). Of the 102 ruptured aneurysms, 85 (83%) were in the anterior circulation and 17 (17%) in the posterior circulation. Of the 58 unruptured aneurysms, 52 (90%) were in the anterior circulation and six (10%) in the posterior circulation.
Aneurysm location
Size of Sac.—
Aneurysmal sac size ranged from 2 to 20 mm. It was less than 5 mm in 39 aneurysms (24%), 5–9 mm in 90 (56%), 10–14 mm in 20 (13%), and 15–20 mm in 11 (7%).
Size of Neck.—
Neck size ranged from 1.2 to 10.0 mm. It was 4 mm or less in 116 aneurysms (72%), and greater than 4 mm in 44 (28%).
Ratio of Sac to Neck Size.—
This ratio was 3 or greater in 35 aneurysms (22%), 3–1.5 in 99 (62%), and less than 1.5 in 26 (16%).
Indication for Aneurysm Treatment
Indication for treatment resulted from a concensus between the neurosurgeon and interventional neuroradiologist. Endovascular therapy was the first-intention treatment, as far as indication was possible. For ruptured aneurysms, the policy was to treat them as soon as possible, generally just after diagnostic cerebral angiography, to prevent aneurysmal rebleeding, and to treat delayed cerebral ischemia due to symptomatic arterial vasospasm by aggressive medication or by endovascular means. For unruptured aneurysms, the aim was to prevent bleeding and improve neurologic symptoms due to mass effect. Whenever possible, all patients and/or their family received complete information before therapy. The treatment took place after they had given their informed consent to participate in the study.
The Micrus Microcoil System
The Micrus microcoil system consists of an embolic microcoil attached to a device positioning unit by a high-tensile-strength polyethylene fiber, which is in contact with a thermic microresistance placed at the distal end of the device positioning unit.
The Micrus microcoils are soft platinum coils with a primary wire diameter of 0.010–0.015 inch. They are either spherical coils (MicruSphere) or helical coils (HeliPaq and extra-soft stretch-resistant UltiPaq) of various diameters and lengths. Spherical coils deploy into a 3D configuration and form an anatomically conformable spherical shape, whereas helical coils deploy into a two-dimensional (2D) configuration.
The Micrus microcoils are detached from the device positioning unit by a thermic process. Electrical energy, supplied by a battery-operated current generator, is converted into thermic energy by the thermic microresistance by applying 7.8 volts for 1 second, immediately followed by 6.5 volts for 4 seconds, at a maximum current of 200 mA. The thermic microresistance, heated to 44.5°C, provides, within 5 seconds, heat shearing of the polyethylene fiber that holds the microcoil to the device positioning unit.
Procedure
All procedures were performed in the angiography room, with the patient under general anesthesia and receiving systemic heparin sodium therapy (Heparin Choay; Sanofi-Synthelabo, Le Plessis-Robinson, France) administered as an intravenous bolus of 50 IU per kilogram of body weight, and then infused continuously to maintain activated partial thromboplastin time at least 3 times the normal level throughout the procedure. Aspirin was added as an intravenous bolus of 100 mg in case of unruptured aneurysms. A four-vessel cerebral angiogram was initially performed and included 3D angiography when available. All the aneurysms were first framed with one or more successive spherical Micrus microcoils of decreasing size, and then filled with helical coils. Coils were packed as densely as possible. The embolization was stopped when angiographically complete occlusion was achieved, when the last coil could not be inserted into the sac, or when occlusion of a normal branch next to the aneurysm might occur. Postoperatively, patients continued heparin therapy for the next 48 hours and/or aspirin therapy with a prescription for 100 mg/day for 1 month.
Data Analysis
Accurate angiographic aneurysmal sac and neck measurements in three orthogonal planes (13) were performed by digital subtraction angiography (3D DSA for 150 aneurysms, and 2D DSA for 10). The sac was classified as small, large, or giant when its maximal diameter was less than 10 mm, 10–24 mm, or 25 mm or larger, respectively. Aneurysms were classified into two groups according to neck size: group A comprised aneurysms with neck size 4 mm or less (n = 116 in 114 patients), and group B comprised those with neck size greater than 4 mm (n = 44 in 43 patients).
The percentage of aneurysmal occlusion at the end of the procedure was evaluated by degree of angiographic occlusion and percentage of volumic occlusion.
Degree of angiographic occlusion as measured with angiography was classified as complete when the sac and neck were densely packed, with no contrast material visible in any projection; near complete when the sac was occluded but with a suspected neck remnant or an obvious tiny neck remnant; and incomplete when there was loose packing and/or persistent opacification of the sac or neck remnant.
Percentage of volumic occlusion indicated the percentage of coil volume occupying the aneurysmal volume. It was calculated by using the following algebraic equation: percentage of volumic occlusion = (volume of the embolized coil) / (volume of the aneurysm). Coil volume was calculated approximately, assuming that the coil was a cylinder. The algebraic equation for the calculation of coil volume was as follows: coil volume = π x [(coil diameter / 2)2 x coil length]. The primary diameter of each type of coil was as provided by the manufacturer (Micrus Corporation). Aneurysm volume was calculated approximately, before embolization, assuming that the aneurysm was ellipsoid: aneurysm volume = 4π/3 x [(d1 / 2) x (d2 / 2) x (d3 / 2)], where d1, d2, and d3 were, respectively, the larger, mean, and smaller diameters of the aneurysmal sac. The larger and smaller diameters were angiographically measured and perpendicular. Mean diameter was calculated by using the following algebraic equation: mean diameter = (larger diameter + smaller diameter) / 2.
Clinical evaluation was performed at the end of the procedure and during hospitalization.
Statistical Analysis
The qualitative and quantitative variables tested consisted of the baseline characteristic variables of the patients and of the aneurysms, and therapeutic and peritherapeutic variables. Baseline characteristics of the patients were age, sex, and clinical findings at presentation (ie, ruptured or unruptured aneurysm). They included WFNS grade on the day of endovascular therapy for patients with ruptured aneurysms. Baseline characteristics of the aneurysms were location, sac and neck sizes, ratio of sac to neck size, and volume. Therapeutic variables were treatment feasibility, technical complications, the number of 3D coils delivered per aneurysm, degree of angiographic occlusion, and percentage of volumic aneurysmal occlusion. Peritherapeutic variables included procedure-related neurologic deficits and overall morbidity and mortality.
Between-group comparisons of therapeutic and peritherapeutic variables were performed by using the Fisher exact test for qualitative variables and the Student’s t test for quantitative variables, with a P value less than .05 considered to indicate a statistically significant difference.
In the entire study population and in groups A and B analyzed as separate strata, independent predictors of therapeutic and peritherapeutic variables were identified by means of a multivariate analysis, using either binary logistic regression (nominal variables) or multiple regression (continuous variables) (LOGXACT; Cytel Software, Cambridge, MA) with a significance level of .05. These predictors were identified among the factors previously screened with univariate analysis by using the Fisher exact test for qualitative variables and the Student’s t test for quantitative variables, with a level of P values less than .2.
Values expressed as means were associated with the standard deviation (± SD) of the mean.
Results
Treatment Feasibility
The treatment proved successful in all 160 aneurysms in 157 patients, but two aneurysms (1%) in two patients were amenable by using a remodeling technique. All 116 aneurysms in 114 patients in group A and 42 (95%) of the 44 aneurysms in 41 patients in group B were treated by using spherical coils positioned first. The two aneurysms (2 [5%] of 44) treated with an adjunctive remodeling technique were both aneurysms with a neck greater than 4 mm. In all, 991 coils were used (303 spherical and 688 helical). To obtain a good frame, a mean of 1.7 spherical coils (range 0–4) in group A and 2.4 (range 1–6) in group B were initially delivered into the aneurysmal sac. A mean of 3.7 helical coils (range 0–17) in group A and of 6.1 (0–18) in group B were added to fill the spaces between the coil loops, to obtain dense coil packing.
Treatment Efficacy
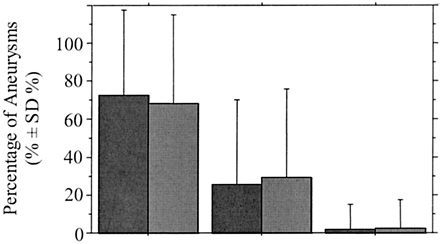
At the end of the procedure, the percentage of aneurysmal occlusion was assessed for all 160 aneurysms in the 157 patients. Degree of angiographic occlusion (Table 3) was classified as complete in 114 aneurysms (71%), near complete in 43 (27%), and incomplete in three (2%). For the 116 aneurysms in group A, degree of angiographic occlusion (Table 4) was classified as complete in 84 aneurysms (72%), near complete in 30 (26%), and incomplete in two (2%). In group B (44 aneurysms, Fig 1), it was classified as complete in 30 (68%), near complete in 13 (30%), and incomplete in one (2%). Degree of angiographic occlusion according to aneurysmal angioarchitecture is shown in Table 4 and Figs 2–4.
Pre- and posttreatment images of an unruptured intracranial aneurysm in a 46-year-old woman who underwent occlusion with 3D Micrus coils.
A and B, Lateral (A) and anteroposterior (B) 3D angiograms of the right internal carotid artery show a periophthalmic aneurysm with a maximum sac size of 9 mm and a neck size of 6 mm.
C and D, Lateral road mapping image (C) and oblique unsubtracted angiogram (D) of the right internal carotid artery after placement of the first spherical coil (9-mm coil loop diameter) show that the 3D configuration of the coil provides an anatomically compliant frame within the aneurysm and a scaffold that covers the neck.
E and F, Lateral (E) and anteroposterior (F) subtracted posttreatment angiograms show complete occlusion of the aneurysm.
Graph shows percentage of aneurysms according to degree of angiographic aneurysmal occlusion at completion of the initial endovascular treatment, in group A ([dark gray bars] aneurysms with a neck ≤ 4 mm) and group B ([medium gray bars] aneurysms with a neck >4 mm). Degree of angiographic occlusion was not significantly different between the two groups (P = .696).
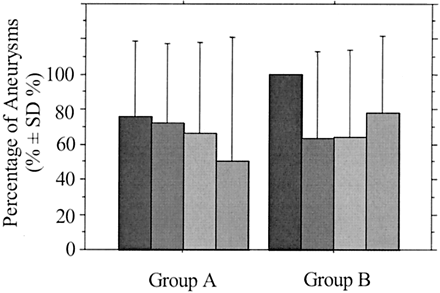
Graph shows percentage of completely occluded aneurysms according to sac size at completion of the initial endovascular treatment in group A (neck ≤ 4 mm) and group B (neck >4 mm). Black bars indicate sac size <5 mm; dark gray bars, sac size 5–10 mm; medium gray bars, sac size 10–15 mm; light gray bars, sac size 15–25 mm.
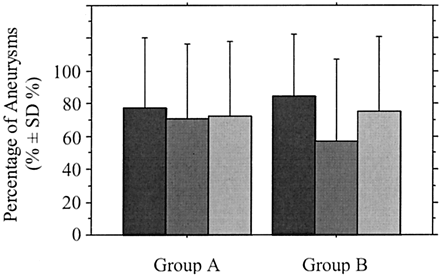
Graph shows percentage of completely occluded aneurysms according to sac-to-neck size ratio at completion of the initial endovascular treatment in group A (neck ≤ 4 mm) and group B (neck >4 mm). Dark gray bars indicate ratio ≥3; medium gray bars, ratio 1.5–3; light gray bars, ratio <1.5.
Mean percentage of volumic occlusion of aneurysms according to degree of angiographic occlusion at completion of the initial endovascular treatment
Degree of angiographic occlusion and mean percentage of volumic occlusion of aneurysms according to aneurysmal sac and neck sizes and the ratio of sac to neck size at completion of the initial endovascular treatment
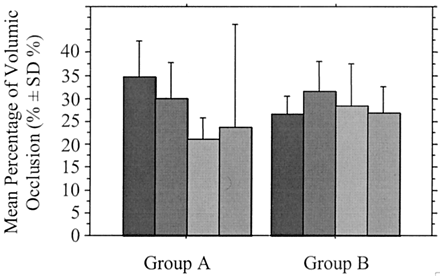
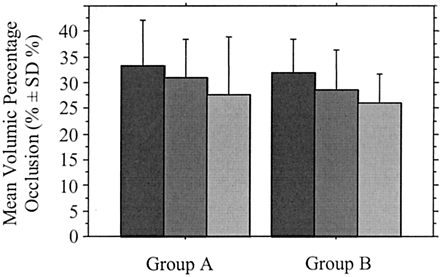
Overall, the mean percentage of volumic occlusion of aneurysms was 30.4 ± 8.2% (Table 3). It was 30.9 ± 8.5% in group A and 29.2 ± 7.2% in group B (Table 4). For aneurysms completely obliterated on the angiograms, the mean percentage of volumic occlusion was 32.4 ± 7.9% in group A and 31.0 ± 6.7% in group B. For near completely occluded aneurysms, the mean percentage of volumic occlusion was 27.8 ± 8.9% in group A and 26.5 ± 6.8% in group B. For aneurysms exhibiting incomplete occlusion on the angiograms, it was 18.2 ± 0.6% in group A and 17.5 ± 0.0% in group B. Mean percentages of volumic occlusion according to aneurysmal angioarchitecture are shown in Table 4 and Figs 5–7. Number of aneurysms according to a line of demarcation of percentage of volumic occlusion drawn at 25% are detailed in Table 5.
Graph shows the mean percentage of volumic aneurysmal occlusion according to degree of angiographic occlusion at completion of the initial endovascular treatment in group A (neck ≤ 4 mm) and group B (neck >4 mm). Percentage of volumic occlusion was not significantly different between the two groups (P = .247). Dark gray bars indicate complete occlusion; medium gray bars, near complete occlusion; light gray bars, incomplete occlusion.
Graph shows the mean percentage of volumic aneurysmal occlusion according to sac size at completion of the initial endovascular treatment in group A (neck ≤ 4 mm) and group B (neck >4 mm). Black bars indicate sac size <5 mm; dark gray bars, sac size 5–10 mm; medium gray bars, sac size 10–15 mm; light gray bars, sac size 15–20 mm.
Graph shows the mean percentage of volumic aneurysmal occlusion according to the sac-to-neck size ratio at completion of the initial endovascular treatment in group A (neck ≤ 4 mm) and group B (neck >4 mm). Dark gray bars indicate ratio ≥3; medium gray bars, ratio 1.5–3; light gray bars, ratio <1.5.
Number of aneurysms completely, near completely, or incompletely occluded according to a demarcation line for percentage of volumic occlusion drawn at 25% at completion of the initial endovascular treatment
Technical Complications
Technical complications were observed in 16 (10%) of the 160 procedures performed to treat 160 aneurysms in 157 patients. These complications were due to the procedure in eight (7%) of the 116 procedures in group A, and two (5%) of the 44 procedures in group B. Complications included thromboembolism in six cases, parent artery occlusion in one case, coil migration in two cases, and coil protrusion in one case. Neither coil rupture nor aneurysm rupture was observed. Technical problems due to the coils were observed in six (0.6%) of the 991 coils used. These problems included nondetachment of coils in five cases (0.5%) and nondeployment in one case (0.1%).
Perioperative Clinical Outcome
All 157 patients with 160 treated aneurysms were clinically evaluated. One hundred forty seven patients (94%) remained clinically unchanged during the perioperative period. Five patients (3%) died: three were classified with WFNS grade 5 and two were classified with WFNS grade 4, and they died because of the severity of their initial hemorrhage. Two patients (1%), respectively classified with WFNS grades 3 and 4, had perioperative neurologic deterioration of delayed cerebral ischemia caused by symptomatic arterial vasospasm. Because of the procedure, two patients (1%) had neurologic deficit due to thromboembolism, and one patient (1%) had intermittent third nerve palsy for 48 hours. Perioperative clinical complications related to the procedure were observed in two patients (2%) in group A and one patient (2%) in group B. The overall morbidity rate was 4% (four patients) in group A and 2% (one patient) in group B. The overall mortality rate was 4% (four patients) in group A and 2% (one patient) in group B. There was no mortality among patients with unruptured aneurysms.
Statistical Analyses
Between groups A and B, no significant differences were noted in treatment feasibility (P = .075), technical complications (P = .238), clinical complications related to the procedure (P > .999), angiographic (P = .696) or volumic (P = .291) aneurysmal occlusion, or overall morbidity or mortality (P > .999 for both). Neither was there any significant between-group difference in percentage of volumic occlusion with the demarcation line at 25% (P = .513).
In the entire study population, patients’ age and sex did not correlate significantly with the immediate anatomic and clinical results. At admission, WFNS grade 5 correlated significantly with mortality (P = .020), and WFNS grade 0 (patients with unruptured aneurysms) correlated inversely with mortality (P = .045). No significant relationships were noted between clinical findings at presentation and immediate anatomic outcome. Aneurysmal features, including location, sac and neck sizes, ratio of sac to neck size, and volume, did not correlate significantly with treatment feasibility, technical complications, or immediate anatomic and clinical results, except for sac size (small versus large) and aneurysmal volume, which correlated significantly with percentage of volumic occlusion (P = .002 and <0.001, respectively), and for sac-to-neck size ratio less than 1.5, which correlated with percentage of volumic occlusion with a relationship of borderline significance (P = .061). When degree of angiographic occlusion was complete, percentage of volumic occlusion correlated significantly with sac size with a relationship of borderline significance (P = .062), but it did not correlate with sac-to-neck size ratio less than 1.5 (P = .147). No significant relationships were noted between procedure-related complications and immediate anatomic and clinical results. Significant differences were noted between the percentage of volumic occlusion of completely and near completely occluded aneurysms (P = .004), and between completely and incompletely occluded aneurysms (P = .002). There was a difference of borderline significance between near completely and incompletely occluded aneurysms (P = .056). The number of 3D coils delivered per aneurysm correlated significantly with percentage of volumic occlusion (P < .001). When three or more 3D coils per aneurysm were used, percentage of volumic occlusion was not significantly different between small and large aneurysms (P = .222). Before the initial procedure, WFNS grade 5 was the only independent predictor of overall mortality (P = .043), according to multivariate analysis. There were no independent predictive factors of technical complications, procedure-related clinical complications, or overall morbidity. Neither were there any independent predictors of immediate angiographic or percentage of volumic occlusion, especially not neck width, sac size, sac-to-neck size ratio, or aneurysm volume. At the end of the initial procedure, percentage of volumic occlusion was the only independent predictor of angiographic complete occlusion (P = .001).
In groups A and B analyzed as separate strata, angiographic complete occlusion correlated significantly with percentage of volumic occlusion (P = .040 and 0.006, respectively). The relationship between mortality and WFNS grade 5 was significant in group A (P = .019), but was not statistically calculable in group B because only one patient died. Aneurysm volume correlated with percentage of volumic occlusion (P < .001 for both). As an independent predictor of overall mortality, WFNS grade 5 was of borderline significance (P = .059) in group A. As an independent predictor of angiographic complete occlusion, percentage of volumic occlusion was significant in groups A and B (P = .007 and 0.065, respectively).
Discussion
The goal of endovascular therapy with coils is to exclude the aneurysmal sac and neck from the intracranial circulation by means of complete and lasting occlusion, while preserving the parent artery and the normal arterial branches next to the aneurysm, without bulging of the coil or slowing of the flow. To prevent delayed coil compaction and subsequent neck recanalization, coils were packed as densely as possible. Incompletely occluded aneurysms (14–22) are at risk of rebleeding and must be considered as cases of treatment failure for as long as they are not retreated.
Several authors (3–9) have studied factors influencing successful angiographic occlusion at initial treatment and reported that complete occlusion was achieved in 59–96% of small-necked aneurysms and 15–50% of wide-necked aneurysms. All these authors pointed out the difficulty of achieving complete obliteration of aneurysms with a wide neck or an unfavorable sac-to-neck ratio, with coils alone at the initial treatment (2). When aneurysms have a small neck, the coils are held inside the aneurysmal sac, thus allowing complete occlusion and dense packing, with a low risk of coil migration or bulging into the parent artery. However, wide-necked aneurysms cannot be densely packed with coils alone, because the neck is too wide to keep the coils inside the aneurysmal lumen, and there is a risk of deposing them in the parent vessel or of their protruding into this vessel once appropriately detached inside the sac. Consequently, only loose packing or incomplete occlusion of these aneurysms can be achieved.
Adjunctive techniques such as the remodeling technique (23), intracranial vascular stent (24, 25), and TriSpan neck-bridge device (26) have been proposed to improve the coil packing and aneurysmal occlusion of wide-necked aneurysms. Three-dimensional coils are one of the newly developed materials designed to bridge the aneurysmal neck with coil loops, thereby facilitating retention of additional coils placed within the aneurysm. A 3D GDC may provide a single-microcatheter solution for the treatment of aneurysms harboring a wide neck or an unfarorable neck-to-dome ratio (10, 11).
In our series, the aneurysms were first framed by deploying one or more successive 3D Micrus coils of decreasing size. Because these coils are soft with a high 3D shape memory, they may provide an anatomically compliant frame within the aneurysm and a stable scaffold that covers the neck. Here, the use of several 3D coils strengthened the stability of the overall frame and provided homogeneous equilateral coverage of the neck. This support allowed us to fill the remaining open spaces, especially in the region of the aneurysmal neck, by using 2D coils packed as densely as possible, so that there was little risk of coil loop protrusion through the neck or of frame deformation.
In our entire study population comprising small and large aneurysms but not giant aneurysms, treatment feasibility and angiographic aneurysmal occlusion were not significantly affected by the baseline characteristics of patients, the occurrence of technical complications, or aneurysmal features. The ability to embolize the aneurysms was not significantly different between groups A and B. Aneurysmal occlusion, by using initial placement of 3D coils, was achieved in 158 (99%) of the 160 aneurysms. Two aneurysms with necks of 8 and 6.2 mm, respectively, and sac-to-neck size ratios of 1.1 and 1.2 were treated by using the remodeling technique. These two aneurysms represented 5% of the 44 aneurysms with a neck greater than 4 mm, and 8% of the 26 with a sac-to-neck size ratio of less than 1.5. Delivery of coils was safe. No marked catheter displacement was observed. No coil displacement was observed after coil detachment, neither was there any displacement of the coil basket during the dense packing of coils. There was no coil rupture or involuntary coil detachment. Detachment of coils was safe. Its duration was consistent and reliable within 5 seconds, because of thermic detachment. Of the 991 microcoils used, nondetachment of five (0.5%) and nondeployment of one (0.1%) were observed. The nondetachment of the coils was due to an inappropriate diameter of the polyethylene fiber in relation to the amount of electrical energy delivered to the thermic resistance. This problem has been the subject of technical improvements. The nondeployment of one coil, 0.010 inch in primary diameter, was due to its bends inside the lumen of a microcatheter-18. Nondeployment was subsequently prevented by using a microcatheter-14 to deliver coils with a primary diameter of 0.015 or 0.010 inch, or a microcatheter-10 to deliver coils 0.010 inch in primary diameter. At completion of the treatment, complete angiographic occlusion was achieved in 72% (84 of 116) and 68% (30 of 44) of aneurysms in groups A and B, respectively. Degree of angiographic occlusion was not significantly different between the two groups.
Percentage of volumic occlusion, which indicates the compactness of coils within the aneurysm, did not correlate with the baseline characteristics of patients, the occurrence of technical complications, or aneurysmal neck size. It was not significantly different between groups A and B. The mean percentages of volumic occlusion were 30.9% and 29.2% in groups A and B, respectively. Satoh et al (27), who examined embolization rates by using aneurysm models constructed of glass tubes, showed that the maximum percentage of volumic occlusion was 32.0–33.3%, even though the aneurysms were packed as tightly as possible with platinum coils. In our series, however, the percentage of volumic occlusion of aneurysms with a sac-to-neck ratio less than 1.5 was lower than that of aneurysms with sac-to-neck ratio 1.5 or greater with a relationship of borderline significance, except when degree of angiographic occlusion was complete. Percentage of volumic occlusion was not related to the neck size provided the sac-to-neck ratio was 1.5 or greater. Adjunctive techniques may be useful for improving treatment feasibility and particularly the percentage of volumic occlusion of aneurysms with a sac-to-neck ratio less than 1.5. Percentage of volumic occlusion of large aneurysms (mean, 26.0 ± 8.5%) was significantly lower than that of small aneurysms (mean, 31.5 ± 7.8%), even when degree of angiographic occlusion was complete with a relationship of borderline significance. One of the reasons may be that the larger the volume of the aneurysm, the greater the length of coil required, and the larger the number of coil intertwinings causing more dead spaces that cannot be filled by the coils. However, the number of 3D coils used per aneurysm correlated significantly with the percentage of volumic occlusion, and may probably result in a homogeneous concentric filling reducing dead spaces between the coil intertwinings. The larger the number of 3D coils used per aneurysm, the better the percentage of volumic occlusion. When three or more 3D coils were first positioned, percentage of volumic occlusion was not significantly different between small and large aneurysms. This may be a means to optimize the packing of coils especially in large aneurysms. In a recent series of 100 cases, Tamatani et al (28) demonstrated that percentage of volumic occlusion was significantly lower in aneurysms with sac size less than 10 mm than that in aneurysms with sac size of 10 mm or more, even when degree of angiographic occlusion at initial treatment was complete. In a series of 152 cases, Debrun et al (29) concluded that the percentage of immediate complete angiographic occlusion was related to the density of coil packing, which in turn was strongly dependent on the geometry of the aneurysm, including the dome-to-neck ratio and neck size.
In our series, before the initial procedure, the morphologic characteristics of aneurysms were not predictive factors of unsatisfactory immediate angiographic or volumic aneurysmal occlusion, according to a multivariate analysis. At completion of the initial procedure, however, percentage of volumic occlusion correlated significantly with degree of angiographic occlusion in groups A and B, and was the only independent predictor of degree of angiographic occlusion. The better the percentage of volumic occlusion, the better the degree of angiographic occlusion.
Degree of angiographic occlusion at the initial treatment is known to be an important predictor of anatomic outcome in endovascular coil occlusion of intracranial aneurysms (9, 28). In a recent study (28), percentage of volumic occlusion was demonstrated to be a more objective and useful index than degree of angiographic occlusion for predicting the long-term anatomic stability of embolized aneurysms. Even when degree of angiographic occlusion after the initial treatment was complete, the frequency of recanalization of embolized aneurysms with coils was significantly lower in aneurysms with a high percentage of volumic occlusion, particularly when it exceeded 25% (28). In such cases, 88% of the aneurysms with a percentage of volumic occlusion of more than 25% were stable at long-term follow-up (28). In our series, the mean percentages of volumic occlusion of the aneurysms initially completely occluded were 32.4 ± 7.9% in group A and 31.0 ± 6.9% in group B. Percentage of volumic occlusion exceeding 25% concerned 82% of the completely occluded aneurysms in group A and 80% of those in group B and was not significantly different between the two groups. This meant that there was no significant difference between these groups as regard prediction of the long-term stability of embolized aneurysms. According to the results for previously published series treated by endovascular aneurysm therapy with coils, revascularization and aneurysm growth ranged from 22.4% to 52.6% and were mainly noted in wide-necked or giant-sac aneurysms, or aneurysms that were incompletely occluded at the initial treatment (3–9, 27–38).
Technical and clinical complications due to the procedure were not related to the baseline characteristics of patients or to the morphologic features of aneurysms. According to a multivariate analysis, no predictor of technical or clinical complications emerged, especially not neck width. Procedure-related complications were not significantly different between groups A and B. Clinical complication rate due to technical complications was 2% in both groups. Overall perioperative mortality correlated significantly with WFNS grade 5 and inversely with WFNS grade 0. There was no perioperative mortality among patients with unruptured aneurysms. Perioperative clinical outcome was not related to age, sex, aneurysmal features, procedure-related complications, or angiographic or volumic aneurysmal occlusion. WFNS grade 5 was the only independent predictor of overall perioperative mortality. No predictor of overall perioperative morbidity was found. There were no significant differences in overall perioperative morbidity or mortality between groups A and B. Perioperative morbidity and mortality rates were 4%, respectively, in group A and 2%, respectively, in group B. In previous series of endovascular treatment with coils, perioperative permanent morbidity and mortality rates due to the procedure ranged from 0–5% and 0–6.4%, respectively (3–9, 27–38).
Conclusion
In our series comprising small and large aneurysms but not giant aneurysms, the use of 3D Micrus coils proved to be useful for improving the coil packing and angiographic and volumic occlusion of aneurysms with a neck greater than 4 mm, at the time of treatment, provided the sac-to-neck ratio was 1.5 or greater, and the largest number of 3D coils were first positioned. Long-term anatomic and clinical follow-up is required to determine the subset of aneurysms in which 3D coils may provide any reliable advantage as regard to safety and efficacy especially in terms of recanalization.
References
- Received May 4, 2003.
- Accepted after revision July 29, 2003.
- Copyright © American Society of Neuroradiology