Abstract
Summary: Spine duplication, which is at the severe end of the split cord malformation, is rarely seen. Radiographic, CT, and MR images of a 15-year-old girl who had lower back pain showed asymmetric lumbar spine duplication with spinal cord tethering secondary to a filum lipoma in the sacrum. Despite gross spinal abnormalities, the patient was neurologically intact and has been followed up with conservative treatment.
Spine duplication is exceptional and is limited to a small number of cases published as case reports in the world literature (1, 2). Although similar cases have been published elsewhere, in this article we report, to the best of our knowledge, the first case of asymmetric duplication of the lumbar spine in a neurologically intact adolescent who had lower back pain.
Case Report
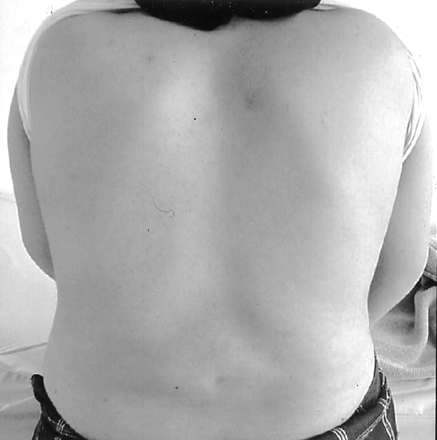
A 15-year-old girl was admitted to the university hospital with the complaint of pain in her lumbar region. The patient was born at full term from a spontaneous vaginal delivery after an uneventful pregnancy. She appeared normal at birth. Her parents were nonconsanguinous, and the maternal history was unremarkable. On physical examination, she appeared normal, and no neurologic deficit was found (Fig 1).
A photograph shows the patient’s thoraco-lumbar alignment to be normal.
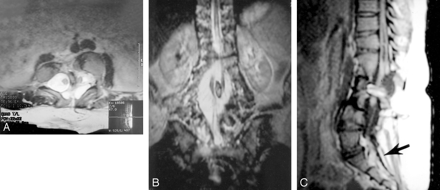
Radiography, CT, and MR imaging were performed. Anteroposterior radiographs of the spine showed spina bifida occulta with a large coronal cleft and complete duplication of L1–L4 lumbar vertebral bodies. Because of the rotation abnormality, the L5 vertebra could not be clearly differentiated from the sacrum (Fig 2). On CT and MR images at levels L1–L4, duplication of vertebral bodies, intervertebral disks, vertebral canal, and transverse processes and narrowed intervertebral disk spaces were demonstrated (Fig 3). At the L5 level, the duplication was seen only in the vertebral body and disk. MR imaging also clarified the intraspinal anatomy, showing a spinal cord split at the L1 level accompanied by hydromyelia along the proximal thoracic vertebrae (Fig 3A). Hemicords were continuous within the two vertebral canals between the L2 and L4 levels (Fig 3B). Conus medullaris appeared at the L4 level, with tethering secondary to a filum lipoma in the sacrum (Fig 3C). The pelvis and hips showed no osseous abnormality. Moreover, there was no evidence of a posterior myelomeningocele or neuroenteric cyst.
Anteroposterior radiograph of the spine shows spina bifida occulta with a large coronal cleft and complete duplication of L1–L4 lumbar vertebral bodies. Because of the rotation abnormality, the L5 vertebra could not be clearly differentiated from sacrum
Axial (A) and coronal (B) T2-weighted and sagittal T1-weighted (C) MR images demonstrate the duplication of lumbar spine and split cord malformation with spinal cord tethering secondary to a filum lipoma (arrow in C).
The patient received conservative therapy for back pain and was monitored closely as an outpatient. Six months after her initial admission to the hospital, her neurologic condition remained unchanged. Because of the absence of neurologic dysfunction, continuous observation of the patient was recommended.
Discussion
Split cord malformations (SCMs) include a wide spectrum of spinal abnormalities, from a simple cleft in the spinal cord known as diastomatomyelia to complete duplication of the spinal column with two hemicords. Almost all reported cases had either severe neurologic malformations (eg, dicephalus, myelomeningocele) or gastrointestinal abnormalities (eg, neuroenteric fistula, omphalocele). Diagnosis of SCMs is becoming more frequent because of modern imaging techniques, particularly with the use of MR imaging in the investigation of young asymptomatic patients with spina bifida occulta (3).
There have been two case reports that were followed up with no surgical intervention, similar to our patient. One, described by Goldberg et al (2), was a neurologically intact 13-year-old girl with a single right kidney and neuroenteric cyst extending from T7 through T10, spina bifida occulta, and partial duplication of the vertebral bodies and disk of L4, L5, and the sacrum. Recently, Ahmed et al (1) reported a case of an asymptomatic and neurologically normal 6-year-old girl who had extensive duplication of the spine from T9 to L5 with a large subcutaneous lipoma, which remained unchanged in size since her birth. In the case of our patient, the extent of spine duplication was between L1 and L5, and physical findings were normal despite spine duplication and vertebral asymmetry on neuroimages. No pathologic mass was found in the spine.
Spinal duplication syndromes are classified by Pang et al (4, 5) into type I and type II. Type I SCM consists of two hemicords, each within its own dural sac and separated by an osteocartilagenous median septum. Type II SCM consists of two hemicords located within a single dural sac and separated by a nonrigid, fibrous median septum. The case presented in the current report appears to belong to SCM type I. The published large series revealed a female predominance and frequent lumbar vertebral region involvement like that of our patient (4, 6).
Pang et al (4, 5) advocate surgery for all patients with type I or type II SCM because of concern about the clinical consequences of the tethering of the cord, which is common in all of these syndromes. Increased risk of neurologic deficits with advancing age and the likelihood that any subsequent surgery is more likely to stabilize rather than to improve the neurologic deficits indicates prophylactic surgery, as advocated by Ersahin et al (6). On the contrary, Ahmed et al and Goldberg et al prefer simple observation in all of their cases in the absence of neurologic dysfunction (1, 2). Similarly, despite the gross malposition of the neural structures and the radiologic evidence of tethering, the clinical condition has not suggested any surgical intervention at 6 months of follow-up in our case, although periodic assessment will be continued in the long run.
- Received April 19, 2003.
- Accepted after revision September 10, 2003.
- Copyright © American Society of Neuroradiology