Abstract
Summary: We present a case of a ruptured giant serpentine aneurysm (GSA) of the superior cerebellar artery in a patient with a Chiari II malformation. The fusiform aneurysm was successfully treated with endovascular parent artery occlusion of the GSA by using detachable coils.
Giant serpentine aneurysms (GSAs) are rare, fusiform aneurysms greater than 2.5 cm, which have a characteristic serpiginous patent channel coursing through a partially thrombosed vascular lumen (1). These aneurysms rarely rupture and more typically cause neurologic insult from mass effect and edema (2). Historically, GSAs have been treated with surgery with or without distal extracranial-intracranial bypass (1). Recently, GSAs have been successfully treated with endovascular parent artery occlusion with or without distal bypass (2).
We present a case of a ruptured GSA involving the superior cerebellar artery (SCA) in a patient with a Chiari II malformation and a congenitally small posterior fossa because of the known Chiari II malformation. The GSA of the SCA was successfully treated with endovascular parent artery occlusion of the GSA.
Case Report
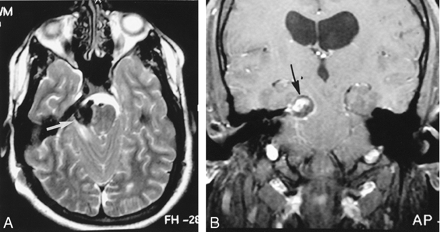
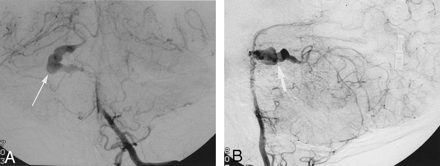
A 21-year-old man male patient with a history of Chiari II malformation and chronic ventriculoperitoneal shunt presented to the emergency room with a severe occipital headache, nausea, vomiting, and an episode of near syncope of 1-day duration. Physical examination revealed no focal neurologic deficits. Cranial CT showed diffuse subarachnoid hemorrhage (SAH) and a 3-cm hyperattenuated mass within the right cerebellopontine angle with mass effect against the midbrain. The patient’s condition was judged to be a Hunt and Hess grade I SAH. An MR imaging study (Fig 1) demonstrated a 3-cm partially thrombosed aneurysm within the right cerebellopontine angle with an eccentrically enhancing patent vascular channel. Cerebral angiography (Fig 2) showed a large, fusiform aneurysm involving the perimesencephalic and ambient portions of the right SCA. The aneurysm demonstrated slow flow, had a serpiginous course, and continued distally to supply the cerebellar territory of the SCA.
T2-weighted (A) and postgadolinium T1-weighted (B) MR images depict the right GSA of the SCA (white arrow). The aneurysm contains a persistent vascular channel, which enhances on the postgadolinium T1-weighted image (black arrow). The vascular channel is surrounded by thrombus. Note the associated mass effect on the midbrain.
Anteroposterior (A) and lateral (B) angiographic images obtained from a left vertebral artery injection show the fusiform serpentine aneurysm of the right SCA. Note that the distal SCA hemispheric branches are supplied by the aneurysm. Also note the slow flow in the right SCA aneurysm with delayed perfusion of distal right SCA hemispheric branches.
Endovascular treatment of this aneurysm was considered, because GSAs have been successfully treated with endovascular parent artery occlusion. The small posterior fossa secondary to the patient’s Chiari II malformation was a complicating matter in this patient. Brain stem symptomatology has been reported after coil placement of a large posterior fossa aneurysm from swelling and mass effect (3). To reduce the risk of swelling, a 25-mg intravenous bolus of dexamethasone (Decadron; Merck & Co., West Point, PA) was administered approximately 1 hour before coil placement, which was followed by a postoperative 7-day steroid taper. In addition, neurosurgical emergency backup was available for evacuation of the aneurysm if the patient’s condition deteriorated after coil placement.
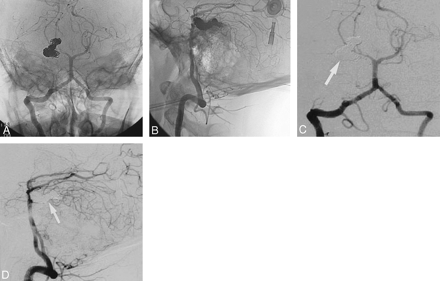
Embolization was performed with the patient under general anesthesia (Fig 3A-D). A 6F vascular sheath was placed within the right common femoral artery. A 6F Cordis Envoy catheter (Cordis, Miami, FL) was placed within the C2 portion of the left vertebral artery. A Prowler-14 microcatheter (Cordis Neurovascular, Miami, FL) was used over a 0.014-inch FasDasher wire (Boston Scientific, Maple Grove, MN) to select the right SCA. Using a roadmap technique, the microcather was advanced to the distal portion of the serpentine aneurysm. Sequentially, a total of 24 Guglielmi detachable coils (GDCs; Target Therapeutics, Fremont, CA) were used to occlude permanently the SCA GSA from a distal to proximal direction. A follow-up angiogram revealed complete occlusion of the SCA and the serpentine aneurysm. On delayed venous phase, the right SCA cerebellar circulation was reconstituted via leptomenigeal collaterals from the ipsilateral right posterior inferior cerebellar artery.
Endovascular occlusion of the GSA of the right SCA. A Prowler-14 microcatheter and Transcend wire were deployed coaxially through a 5F Cordis Envoy placed in the left vertebral artery at the C2 level. The aneurysm was subsequently coiled with 24 GDCs distally to proximally following an intravenous steroid bolus.
Unsubtracted (A and B) and subtracted (C and D) anteroposterior and lateral angiographic images after coil placement demonstrate a dense coil mass approximating the serpentine shape of the residual channel of the aneurysm. Note that the SCA aneurysm and the proximal parent SCA is completely occluded (arrows).
The patient awoke from general anesthesia with transient right cranial nerve VI palsy. One week after the procedure, the patient developed transient hiccups and a mild right-hand ataxia. Repeat cranial CT was unrevealing, which was probably because of coil artifact. Repeat angiography was performed and findings were unchanged. The patient’s right-hand ataxia resolved with physical therapy at 2-month follow-up.
Discussion
GSAs are defined as giant (>2.5 cm), fusiform aneurysms that are partially thrombosed (1). These aneurysms typically have a residual patent channel located within a partially clotted lumen, which is not endothelialized and lacks a normal media and elastic lamina (1). As fusiform aneurysms, GSAs have no definable neck and continue distally to supply the normal arterial supply of the parent artery. The clinical presentation of GSAs is usually produced by mass effect, and adjacent edema and is rarely caused by rupture of the aneurysm resulting in SAH (4).
GSAs have characteristic imaging findings at CT, MR imaging, and angiography. CT usually demonstrates a well-circumscribed hyperattenuated mass associated with varying degrees of edema (2). MR imaging findings typically consist of a mass with heterogeneous signal intensity characteristics that represent various stages of hemoglobin degradation products along the walls of the lumen and a flow void representing the patent channel within the aneurysm (2). The residual channel within the partially thrombosed lumen may demonstrate enhancement at MR imaging (5) that is probably secondary to the slow flow within the aneurysm. Cerebral angiography (2, 4) demonstrates a giant fusiform aneurysm with a characteristic serpiginous channel, which supplies the normal distal circulation of the parent artery. There is typically slow flow through the channel of the GSA. The adjacent partially thrombosed lumen may be inferred from an avascular mass surrounding the patent channel.
The treatment of GSAs should eliminate the patent vascular channel, halt the growth of the aneurysm, and relieve the adjacent mass effect (2). Historically, this was performed with total surgical excision, carotid ligation, entrapment, or aneurysm wrapping (1, 6). More recently, endovascular therapy has been performed with significant success (2). GSAs have been treated effectively with parent artery occlusion with detachable balloons, GDC coils, or n-butylcyanoacrylate (2). High-dose corticosteroids have also been advocated before treatment of GSAs for reducing the mass effect related to the aneurysm and reducing the risk of neurologic insult (2).
Our patient with a known Chiari II malformation had a previously unreported GSA involving the SCA that presented with SAH. Complicating matters in this patient was a congenitally small posterior fossa related to the patient’s Chiari II malformation. Aggravation of brain stem symptoms has been reported after coil embolization of a 20-mm SCA aneurysm (3). Therefore, endovascular parent artery occlusion of the GSA was performed with great caution after a high-dose corticosteroid bolus (Decadron 25 mg intravenously) with a tapering steroid dose after the procedure to reduce the mass effect related to the aneurysm after coil placement. Parent artery occlusion of the superior cerebellar artery is typically well tolerated (7). Therefore, we occluded our patient’s SCA without initially performing a balloon occlusion test and without considering a surgical bypass procedure. The patient had mild, transient cranial nerve VI palsy immediately after the procedure. The patient’s transient hiccups occurred 1 week after embolization and were probably related to posterior inferior cerebellar artery ischemia perhaps due to delayed mass effect. The patient’s delayed mild right-hand ataxia was probably related to SCA ischemia after parent artery occlusion. This mild ataxia resolved completely after 2 months of rehabilitation. There were no permanent neurologic sequelae from the endovasular treatment of the serpentine aneurysm.
Conclusion
We report the case of a Chiari II malformation with a ruptured GSA involving the SCA. The patient was successfully treated with endovascular coil occlusion of the GSA and the parent SCA.
Footnotes
Presented at the 40th annual meeting of the American Society of Neuroradiology, Vancouver, BC, May 11–17, 2002.
- Received June 5, 2003.
- Accepted after revision September 23, 2003.
- Copyright © American Society of Neuroradiology