Abstract
BACKGROUND AND PURPOSE: A reversible decrease in brain size has been demonstrated during normal pregnancy that is maximal at term and returns to normal after many months. The purpose of this longitudinal study was to use phosphorus-31 MR spectroscopy to determine if metabolic changes explain this physiologic event.
METHODS: Pregnant women (n = 12) were examined at term and up to 6 months after delivery. Nonpregnant control subjects (n = 7) were imaged twice (a month apart) to exclude hormone effects. Brain 31P MR spectra were acquired at 1.5 T, and intracellular pH was calculated from the chemical shift between phosphocreatine and inorganic phosphate resonances. Statistical analysis was performed by using an analysis of variance.
RESULTS: We found no statistically significant differences in the relative levels of metabolite associated with cerebral bioenergetics and cell membrane metabolism between pregnant women and nonpregnant women. However, a significant increase in cerebral pH was observed in pregnant women at 6 weeks after delivery compared with control subjects (7.074 ± 0.063 vs 7.017 ± 0.041; P < .05). pH returned to normal by 6 months after delivery (7.014 ± 0.010).
CONCLUSION: Changes in brain size associated with pregnancy appear to be associated with an increase in intracellular pH after delivery. The observed alkalosis may reflect altered cellular metabolism. These persistent brain perturbations associated with pregnancy indicate that, when postpartum physiologic and pharmacologic changes are measured, long-term effects may be expected in central nervous system processing.
A reversible decrease in brain size in pregnancy that has a prolonged recovery is reported (1). The normal sequence of change is a progressive decrease in brain size during pregnancy up to the time of delivery, followed by an increase in size for as long as 6 months. A similar sequence, but one of greater magnitude, has been observed in women with mild or severe preeclampsia (1). The changes take months to return to normal after delivery. On quantitation by using contour and thresholding technique (2), highly significant changes occur: The lateral and third ventricular volume increases at term by up to 30%, and brain size decreases by as much as 6% from about 20 weeks of gestation—about the time of placental implantation—and then returns to normal at about 6 months.
Rutherford et al (3) have reported relative cerebral ischemia in preeclampsia compared with healthy pregnancy. The mechanism and functional relevance is speculative and may be related to hormonal (4), vascular (5) and metabolic (6) disturbances. This present study was designed to investigate the metabolic changes that may be part of this normal adaptation to parturition.
Phosphorus-31 MR spectroscopy provides in vivo information about the relative concentrations of neurochemicals, particularly membrane phospholipids and high-energy phosphates (7). Although it has relatively low spatial resolution, our previously reported MR imaging findings (1) of diffuse global changes suggest that this technique is appropriate for defining normal cerebral metabolism and its potential perturbation in parturition. Hence, the aim of this study was to assess changes in brain metabolism by means of in vivo 31P MR spectroscopy in a longitudinal design involving pregnant women and a control group of nonpregnant women.
Methods
Patients and Control Subjects
Approval was obtained from the ethics committee of our institutional review board, and participants provided written informed consent. Pregnant women (n = 12) were scanned at term, at 6 weeks, and (if possible) at 6 months after delivery. For all imaging studies performed during pregnancy, the abdomen and pelvis were positioned with the left side down to avoid occlusion of the inferior vena cava; this position was maintained for the duration of the scan, which was about 30 minutes. Nonpregnant women (n = 7) were included as control subjects; they were scanned twice, a month apart, to exclude sex-hormone-related effects.
31P MR Spectroscopy
31P MR spectra were acquired, as previously described (8). Briefly, all measurements were obtained by using a 1.5-T MR imaging unit (Eclipse; Philips Medical Systems, Cleveland, OH). T1-weighted spin-echo axial MR images were acquired to define the position of the volume of interest. Localized 31P MR spectra (70 × 70 × 70–mm voxel; TR = 10 seconds; 64 signal intensity averages) were acquired by using an image-selected in vivo spectroscopy sequence (9). Figure 1 is an axial image (TR/TE = 646/20) showing typical voxel placement in the brain. Given the large voxel required for 31P MR spectroscopy, no attempt could be made to localize a particular region of the brain.
Axial T1-weighted image (646/20) shows typical placement of the 70 × 70 × 70-mm voxel inside the brain.
Spectra Analysis
A single observer (G.H.) blinded to the clinical status of the subjects analyzed the MR spectra by using prior knowledge in the Algorithm for Magnetic Resonance algorithm (10) included in the Magnetic Resonance User Interface software program (11). Full details of the analysis technique and prior knowledge used to fit the spectra have been previously described (12). Peak areas for phosphomonoester (PME), inorganic phosphate (Pi), phosphodiester (PDE), phosphocreatine (PCr), and three nucleoside triphosphates (NTPs; γ, α, and β) were calculated as a percentage of the total peak areas, or the fraction of the peak area compared with the sum of all the peak areas of all narrow resonances (Table).
Results from the spectral analysis
In Vivo pH Calculation
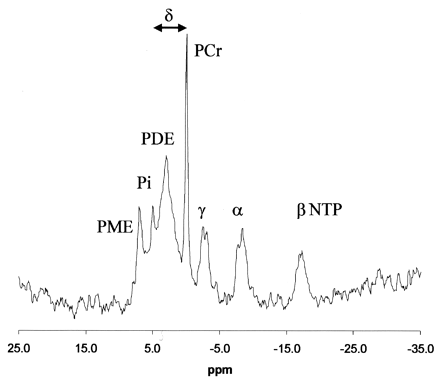
Intracellular pH (pHi) was measured by using the chemical shift difference between the Pi and PCr peaks and the Henderson-Hasselbalch equation in the form published by Petroff et al (13): pHi = 6.77+ log [(A − 3.29)/(5.68 − A)], where A = chemical shift difference in ppm between Pi and PCr (Fig 2).
Localized brain 31P MR spectrum and fit of the data produced by spectral analysis. Arrow indicates the chemical shift between PCr and Pi peaks measured to determine the pHi.
Descriptive and comparative data analysis was performed by using the SPSS software (version 11 for Windows; SPSS, Chicago, IL). We also conducted a one-way analysis of variance using multiple comparisons and post-hoc tests to compare findings in pregnant women with those of the control group.
Results
Twelve pregnant women participated in this study, completing the term and 6 week scans. Three were unavailable for the 6-month scan. All but two were breastfeeding at 6 weeks. The nonpregnant control group consisted of seven age-matched female volunteers. The mean (SD) ages of the women were 33 (4) years in the pregnant and 28 (6) years in the control group; these were not statistically different.
Figure 2 shows a typical brain 31P spectrum from a volunteer. The chemical shift of PCr was set to 0 ppm in accordance with general conventions. Peaks for PME; Pi; PDE; PCr; and γ, α, and β NTP are shown. The three NTP peaks consisted of signals from mainly adenosine triphosphate, although there were contributions from other diphosphate and triphosphate resonances (14). Phospholipid cell membrane precursors, including phosphocholine (PC) and phosphorylethanolamine (PE) and sugar phosphates contributed to the PME resonance, while phospholipid cell membrane degradation products, including glycerophosphorylcholine (GPC) and glycerophosphorylethanolamine (GPE), contributed to the PDE resonance.
The Table shows results from the spectral analysis for the individual phosphates and their ratio. The pregnant women and the control group had no significant differences in the relative levels of any of the cerebral metabolites except for a small, yet significant, increase in brain pHi. One-way unrelated analysis of variance for pH showed a significant effect between the groups (P = .022). A post-hoc analysis using the LSD test for multiple comparisons showed that women who were at 6 weeks after delivery had a higher pHi than that observed all other times (P < .05).
Discussion
During parturition, women are at greater risk of cerebral events than in the nonpregnant state, as a result of medical diseases of pregnancy such as preeclampsia (3) or thromboembolic or other vascular diseases. Their vulnerability to these disorders may be related to underlying changes in physiological characteristics induced by the pregnant state. For example, we have observed that the magnitude of the reversible decreases in brain size during pregnancy is increased in preeclampsia (1). Although the mechanisms for these changes are still speculative, the reporting of ischemic changes during preeclampsia suggests that a metabolic component may have functional importance. The presence and extent of metabolic changes in the brain of pregnant women may be determined noninvasively by using MR spectroscopy.
Previous studies have shown that the postpartum brain increases in size and that the return to prepregnant values is slow, taking months rather than days (1). Several potential mechanisms have been postulated to explain this observation, including the fact that the nutritional needs of the fetus in utero may be met by accessing the maternal tissues stores, including some membrane constituents. Studies of blood concentrations of fatty acids, cholesterol, and phospholipids in mother and baby would test this hypothesis (15). If similar changes in brain cellular composition occur, cell membrane metabolic studies should help to elucidate them. The phospholipid precursor PE and the membrane degradation product GPC are thought to be of particular significance, and they are also major contributors to the PME and PDE signal intensity in the 31P MR spectra of brain, respectively.
We postulated that parturition may induce prolonged brain metabolic perturbations. Our data for control subjects can be compared with MR spectroscopic results of other healthy adults. In a mixed female–male group of 15 volunteers, the mean ± SD values for pH ranged from 7.08 ± 0.04 in the posterior brain to 7.07 ± 0.03 for the whole brain, and no specific regional variations in pH were noted (16). These results are similar to our postpartum values and would have been considered normal had a further time frame not been added to our pregnant group, plus a separate female control group. These additional groups proved that the 6-week follow-up data were not an accurate reflection of normal values.
One of the major difficulties with 31P MR spectroscopic studies is the comparison of results between centers. This particularly affects peak areas; even if variations in pulse sequence design and hardware caused no differences, variations in analytic techniques can produce large discrepancies in these measurements (12). Furthermore, despite the ease of calculation, reported pH values vary greatly, as measured by different groups (17).
One study of brain tumors combined the results of 36 healthy men and women (18). Volumes of interest were centered on the parieto-occipital region of the brain. The mean pH was 7.04, and alkalinization to 7.09 and higher accompanied the findings of patients with brain tumors. This alkalinization was explained by an accumulation of catabolites after cellular proliferation and was considered to result from stimulants (such as vasopressin) activating Na+/H+ exchange. In a study of schizophrenic patients and 19 control subjects (19), values from the frontal lobe in healthy men and women were acidic, with pH means of 6.98 and 6.99, respectively. The other spectra (e.g., PCr and Pi) exhibited sex differences. These differences were considered to provide a basis for sex differences in neuroleptic drug activity, since the changes were not observed in the patients. With regard to the pH results and the different regions of interest used in these studies, the varying percentages of white matter and gray matter might have contributed to the measured differences. Tissues in each regions of interest are not be homogeneous and within tissues, neurons and their supporting structures (astrocytes) maintain different basal pH values of 6.95 and 7.05, respectively (20). These results may explain why different pH values are observed, even in healthy control subjects, and why maintaining a closely defined region of interest is crucial for cross-sectional and longitudinal studies. In our study, the region of interest included the basal ganglia and the posterior parietal region, as these areas are highly susceptible to physiologic alterations (21). The automated computer analysis ensured a high degree of objectivity and reproducibility that was not dependent on observer responses.
Pregnancy is associated with mild alkalemia secondary to hyperventilation contributing to a respiratory alkalosis (22). The blood pH is buffered through renal compensation and decreased bicarbonate levels (23). However, within red blood cells, intracellular acidosis has been demonstrated (24). Thus, oxygen delivery may be facilitated and possibly central nervous system respiratory stimulation. In the brain, other acid-base changes occur during pregnancy; these include an increase in CSF alkalinity with pH values of 7.33 in the third trimester at delivery compared with 7.30 in nonpregnant control subjects (25). Quantitative models to explain these alterations include Stewart’s approach (26). Wolfe et al (27) modernized this computer prediction to include hypothalamic-pituitary-adrenal (HPA)-axis endocrine control during pregnancy (e.g., vasopressin activity). One of its basic variables is a reduced CSF volume in pregnancy. The usefulness of such models are only as accurate as compartmental size; therefore, the present results not only supports the development of better models but also contributes to improved multicompartmental models for the overall acid-base status in the area of interest. In the future, pHi and extracellular pH may be determined with a refinement in techniques (20).
Large increases in the secretion of sex steroid hormones and the secretion of peptide hormones are unique to childbearing. They can change several aspects of brain function, and metabolic and fluid adjustments may be adaptive to cope with postdelivery behavior. In particular, the role of the HPA axis needs further investigation because it possibly acts as a coordinator (28). The persistence of physiologic changes into the postpartum period corresponds with findings of acid-base balance in parturition and a continuation of the respiratory alkalosis being observed soon after delivery (29). However, its persistence as long as 6 weeks after delivery has not been recorded, to our knowledge. In this present study of lactating and nonlactating women, pHi in the brain tissue was statistically significantly more alkaline at 6 weeks after delivery than during pregnancy or in female controls. pHi is a fundamental parameter in the regulation of energy metabolism and cellular activity. Metabolic changes continued until more than 6 weeks after delivery, and long-term follow-up in the postpartum period with or without lactation is critical to physiologic and pharmacologic observations.
Conclusion
In the healthy parturient and nonpregnant women examined, the only statistically significant metabolic effect measured to relate to changes in brain size was a decrease in intracellular acidosis at 6 weeks after delivery. Although the relative levels of membrane phospholipids did not change significantly, brain changes naturally persistent in the long term.
Acknowledgments
We thank all of the women who took part in this study and traveled long distances for follow-up scans and Muriel Dockendorff por inspiratio. We also thank the MRC for its financial support.
References
- Received February 26, 2004.
- Accepted after revision May 12, 2004.
- Copyright © American Society of Neuroradiology