Abstract
Summary: Post-transplant lymphoproliferative disorder complicates approximately 1% of all renal transplants (1). The usual site of occurrence is within the abdomen, thorax, allograft, or head and neck. Central nervous system involvement is uncommon but, when present, occurs in isolation, sparing other organ systems. Few articles in the radiology literature have focused on the acute and follow-up central nervous system findings of the disease, especially in children. Because the clinical and imaging characteristics of central nervous system post-transplant lymphoproliferative disorder overlap those of infection and primary central nervous system lymphoma and the fact that untreated posttransplant lymphoproliferative disorder has a poor prognosis, it is important to maintain a high index of suspicion for this disorder so that appropriate treatment can be instituted.
Post-transplant lymphoproliferative disorder is a recognized complication of organ transplantation, which has been well described in the literature. The central nervous system is an unusual site of occurrence, and, to our knowledge, little has been written about posttransplant lymphoproliferative disorder in this location, particularly in children. The purpose of this case description is to demonstrate and discuss the acute and chronic imaging findings of central nervous system posttransplant lymphoproliferative disorder.
Case Report
A 6-year-old-girl with VATER syndrome, who had undergone renal transplantation at 3 years of age presented to the emergency department with a 6-week history of intermittent headaches, nausea, and vomiting. Her headaches had increased in frequency and severity and were now waking her from sleep. On the day of admission, she experienced a new onset of seizures. MR imaging was performed and revealed multiple lesions in the cerebral hemispheres involving the cortex and the deep white matter, some of which were enhancing (Fig 1). At that time, infection was thought the most likely cause. Fungal infection was favored over bacterial, in light of the clinical course and absence of fever. Brain biopsy was preformed and was diagnostic of post-transplant lymphoproliferative disorder of intermediate grade (diffuse large cell monomorphic type). The cells showed Epstein-Barr virus–latent membrane protein immunoreactivity. CT of the chest, abdomen, and pelvis, as well as nuclear medicine bone scanning, revealed no additional evidence of extracranial posttransplant lymphoproliferative disorder.
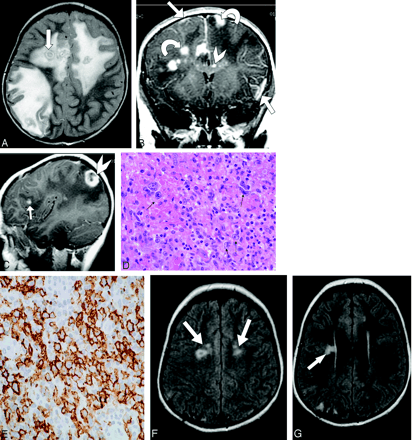
Post-transplant lymphoproliferative disorder in a 6-year-old girl with new-onset seizures and worsening headaches 3 years after renal transplantation.
A, Axial T2-weighted image (TR/TE, 4000/97.28) shows bilateral hyperintense brain lesions, some of which are ringlike (arrow) and have extensive surrounding vasogenic edema.
B, Coronal T1-weighted image (TR/TE, 500/8), obtained after gadolinium administration, reveals bilateral enhancing foci in cerebral hemispheres (curved arrows) and corpus callosum (arrowhead), with patchy leptomeningeal enhancement (straight arrows).
C, Sagittal T1-weighted image obtained after gadolinium administration demonstrates ring enhancement in some of the larger foci (arrowhead) and enhancement in the smaller foci (arrow).
D, Microscopic specimen obtained at biopsy reveals diffuse large cell lymphoma (monomorphic post-transplant lymphoproliferative disorder) and tumor cells with prominent nucleoli and irregular nuclear contours (arrows) (hematoxylin and eosin, original magnification ×40).
E, Microscopic specimen shows that the large dysplastic cells express the B-cell marker (CD20 antibody stain [Dako Corporation, Carpenteria, CA], original magnification ×40).
F, G, Axial fluid-attenuated inversion recovery images (TR/TE, 8402/150), obtained 26 months after initial diagnosis, show resolution of most of the abnormal T2 hyperintensity with only small areas of abnormal signal intensity remaining (arrows), none of which enhanced.
Post-transplant lymphoproliferative disorder was treated with reduction of immune suppression (a lower dose of cyclosporine) and by intravenous acyclovir. Phenobarbital was used for seizure control. The reduction in immunosuppression was tolerated well without evidence of transplant failure or graft-versus-host disease. Her headaches resolved, and she experienced no further seizures. MR imaging showed dramatic improvement over the next 3 months with complete resolution of the smaller lesions, marked reduction in size of the remainder, and resolution of contrast enhancement. The last images were obtained 26 months after initial diagnosis, and none of the lesions enhanced at this time. MR imaging remained stable thereafter.
Discussion
Post-transplant lymphoproliferative disorder represents a spectrum of lymphoid hyperproliferation, ranging from benign hyperplasia to lymphoma. It occurs in patients who have undergone solid organ or stem cell transplantation and are on an immunosuppressive regimen to prevent rejection. Lymphoid growth proceeds in an uncontrolled fashion as a result of the immunocompromised state. Epstein-Barr virus infection or reactivation is thought to play a role, and Epstein-Barr virus seronegativity at the time of transplant is a well-known risk factor for developing posttransplant lymphoproliferative disorder. Additional risk factors include pediatric age, allograft type, specific immunosuppressive regimen, and concomitant cytomegalovirus infection (1–4). Post-transplant lymphoproliferative disorder is 4 times more common in children than in adults, likely due to Epstein-Barr virus seronegativity in children (4).
The overall frequency of post-transplant lymphoproliferative disorder has been reported to range between 1% and 10% (1, 4, 5). Renal transplant recipients generally have lower risks (≈1%) than recipients of liver, lung, and heart transplants; this discrepancy may be attributable to differences in the immunosuppressive regimens (1, 5, 6). Most cases of post-transplant lymphoproliferative disorder occur within the first 2 years following transplantation (2, 3, 5). Central nervous system involvement is relatively uncommon today. A review by Miller et al (5) found that with the introduction of newer, more potent immunosuppressants, such as cyclosporine, there has been a shift in sites of involvement. When azathioprine was the primary immunosuppressant, the central nervous system was the predominant site of involvement. With the introduction of cyclosporine, however, isolated central nervous system involvement has become relatively uncommon. Instead, typical sites of involvement today are the thorax, abdomen, and even the allograft (5, 7). When post-transplant lymphoproliferative disorder does involve the central nervous system, it usually does so in isolation without evidence of the disorder in other organ systems (8, 9). In addition, a recent review of a small series of patients with central nervous system post-transplant lymphoproliferative disorder found that the higher grade monomorphic type tends to be the rule (8).
Multiple ring-enhancing lesions with surrounding edema, as seen in our case, are the most common findings on CT and MR imaging (8, 9). The appearance is similar to that of central nervous system lymphoma in patients with AIDS or infection and primary central nervous system lymphoma in immunocompromised patients, in whom ≤80% are multifocal (8, 9). Distinguishing between post-transplant lymphoproliferative disorder, primary central nervous system lymphoma, and infection cannot be based on imaging findings but requires a tissue biopsy; however, adequate distinction between central nervous system post-transplant lymphoproliferative disorder and primary central nervous system lymphoma is often possible, based on the clinical history and response to therapy (8).
The primary treatment for post-transplant lymphoproliferative disorder is reduced immunosuppression. Antiviral therapies are often added to control Epstein-Barr virus replication, and cytokines have also been used to boost the immune system and control viral load. The utility of these agents is not yet clear because to our knowledge, there have been no large-scale investigations to confirm their success. When initial treatment regimens fail, chemotherapy is often begun (2, 5). Anti-B-cell antibodies are a promising option that is under investigation. Initial results have shown resolution of disease in many patients. Those with multiorgan involvement, central nervous system involvement, and late-onset post-transplant lymphoproliferative disorder, however, have shown little response (2).
The prognosis for patients with post-transplant lymphoproliferative disorder varies according to the age of the patient and the extent of disease. Children, those with localized disease, and those with polymorphic rather than monomorphic disease have better survival rates (3, 5, 7). Surveillance for Epstein-Barr virus activity using antibody titers or polymerase chain reaction may enable early identification of at-risk patients (2, 4). Prevention of post-transplant lymphoproliferative disorder remains a challenge. Prophylaxis with antiviral agents has been by used by many centers, but the overall effectiveness is controversial. Although these agents inhibit the replication of Epstein-Barr virus, they do not eradicate the latent virus (2, 4). Large-scale studies are needed to assess their utility.
In light of the variety of clinical and imaging manifestations of post-transplant lymphoproliferative disorder and the many diagnoses it can mimic, it is important to maintain a high index of suspicion so that appropriate treatment can be started before disease progression occurs. If treated early, long-term outcome is good.
Footnotes
Presented at the annual meeting of the American Society of Neuroradiology, Seattle, June 2004.
References
- Received August 9, 2004.
- Accepted after revision October 12, 2004.
- Copyright © American Society of Neuroradiology