Abstract
BACKGROUND AND PURPOSE: Although attenuated coil packing of intracranial aneurysms is an important goal of endovascular embolization, because of their small size, some aneurysms can only be treated with a single embolization coil. We retrospectively analyzed small aneurysms treated with a single Guglielmi detachable coil (GDC) to determine whether the coil-embolization ratio (CER) is predictive of embolization stability.
METHODS: The CER was determined for 25 small (<7-mm diameter) intracranial aneurysms, each treated with a single embolization coil. The largest aneurysm dimension, estimated by comparison to anatomic landmarks, was used for volume calculation based on a spherical model. Coil volumes were according to manufacturer specifications. CER was calculated by the formula (coil volume/aneurysm volume) × 100%. Embolization stability was assessed by angiographic follow-up.
RESULTS: The average CER for all aneurysms was 8.2% (SD, 6.5%; range, 0.6%–21.1%). Twelve percent of the aneurysms had a CER >20%. Follow-up angiographic assessment was conducted at an average of 30.8 months after initial treatment. Eighty-four percent of the aneurysms were obliterated. One large (6 × 10 mm) and 3 small (<1 mm) recurrences were identified. The average CER for unchanged aneurysms was 8.0% (SD, 5.9%) and for the recurrent aneurysms was 8.8% (SD, 8.7%), which was not statistically significant.
CONCLUSION: Small aneurysms treated with a single coil achieved satisfactory stability despite having a low average packing attenuation. CER was not predictive of recurrence in small intracranial aneurysms treated with a single detachable coil.
Occurring in as much as 8% of the population, intracranial aneurysms are a common vascular abnormality that can result in disability or death (1). Sequelae develop because of mass effect upon adjacent neural tissue or because of rupture. Treatment is directed at the prevention of rupture or relief of mass effect. Surgical clipping was the only available treatment until endovascular coil embolization became available at many centers in 1995. Advantages of coil treatment include speed of procedure, use of an endovascular approach (rather than craniotomy), and ability to treat multiple aneurysms at the same time (2, 3). Coil compaction and aneurysm remnants are among the disadvantages of coil treatment. Allowing persistence of the aneurysm lumen, compaction places the patient at risk for subarachnoid hemorrhage. Dense packing of aneurysms with coil material helps prevent the coil compaction (4).
The correlation between embolized volume and stability of embolized aneurysms may be significant (4–8). Calculating a coil-embolization ratio (CER) quantifies the amount of coil material occupying the aneurysm, providing an objective measurement of aneurysm obliteration. In combination with subjective angiographic assessment, this percentage of embolized volume may allow more accurate prediction of aneurysm recurrence. By establishing a preprocedural packing attenuation goal, the ratio may also help determine which coil lengths and types would be most efficacious.
Previous studies have established minimum suggested packing attenuation thresholds by evaluating aneurysms of all sizes (4–8). In many patients with small aneurysms, it is often not possible to place more than a single coil. The usefulness of the CER in predicting the stability of a subset of small aneurysms treated with a single detachable coil has not been determined. We calculated the CER of small single-coil-embolized aneurysms to determine the usefulness of the measurement in this unique setting.
Methods
Patient Population
Thirty-five consecutive small intracranial aneurysms were treated with a single detachable coil at our institution from August 1997 to May 2004. Ten of these single-coil-embolization aneurysm patients were excluded from the study because of a lack of angiographic follow-up. Four of these 10 patients were treated only recently, and have angiograms scheduled at a later date. Two of the patients had a follow-up MR examination instead of a catheter angiogram. One patient has declined follow-up, because of family illness. Three of the patients were unable to be contacted by phone or mail.
The study population, then, consisted of 25 patients, each with a small intracranial aneurysm treated with a single embolization coil. All patients had a catheter angiogram at the time of presentation and a second angiogram an average of 30.8 months after initial treatment. There were 16 women and 9 men. The mean age of the patients at the time of presentation was 47 years (range, 21–72 years; median, 48 years). Twelve of the patients had additional intracranial aneurysms.
Aneurysm Rupture Status
A total of 15 patients presented with acute subarachnoid hemorrhage, verified by CT and/or CSF analysis. In the setting of acute subarachnoid hemorrhage, a solitary aneurysm was presumed to have ruptured regardless of size or morphology. If multiple aneurysms were present, proximity to subarachnoid blood, dome irregularity, and relatively larger size were factors used to determine aneurysm rupture status. Fourteen of the aneurysms treated with single coils were unruptured. Nine of the aneurysms treated with single coils were thought to have acutely ruptured. We were unable to assign a rupture status to 2 (an anterior communicating and a middle cerebral artery) aneurysms because of a lack of distinguishing radiographic features in the setting of multiple aneurysms.
Aneurysm Location
Aneurysm location was assigned as per standard convention. Twenty-one aneurysms (84%) were in the anterior circulation and 4 (16%) were in the posterior circulation. Of the 9 previously ruptured aneurysms, 7 (78%) were present in the anterior circulation and 2 (22%) were present in the posterior circulation. Of the 14 unruptured aneurysms, 12 (86%) were present in the anterior circulation and 2 (14%) were present in the posterior circulation. The two aneurysms of unknown rupture status were present in the anterior circulation.
Aneurysm Measurements
Aneurysm measurements were made at the time of initial angiography by comparing to the estimated size of internal controls such as the internal carotid artery or the basilar artery. Because these aneurysms were so small, they were nearly spherical in shape. Therefore, the formula V = 4/3 × p × r3 was used to calculate the aneurysm volume; however, the largest aneurysm dimension, as estimated by comparison to anatomic landmarks, was used for volume calculation. This may have led to overcalculation of the aneurysm volume and therefore lower CER. The average aneurysm was 3.5 mm (SD, 1.46 mm; range, 2–7 mm) in maximum diameter. Average aneurysm volume was 34.8 mm3 (SD, 48.7 mm3). Coil volumes were according to manufacturer specification, ranging from 0.41 mm3 to 3.83 mm3 (mean, 1.2 mm3; SD, 0.8 mm3). CER was calculated by the formula (coil volume/aneurysm volume) × 100%.
Aneurysm Surveillance
An initial catheter angiogram was performed at the time of presentation. Routine angiographic follow-up for coiled aneurysms is at 6 months and 24 months at our institution. For this study, the last angiographic assessment after initial treatment was used. This resulted in an average 30.8 ± 31.8 months angiographic follow-up. New aneurysm filling on the follow-up examination was considered to be a recurrence.
Results
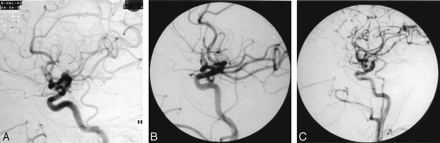
Twenty-one of the 25 small aneurysms (84%) were completely obliterated with a single coil (Fig 1).
Stable aneurysm, patient 14, a 55-year-old man with multiple nonruptured aneurysms.
A, Lateral right common carotid arteriogram demonstrating a 4-mm posterior communicating artery region aneurysm and a carotid bifurcation aneurysm.
B, Lateral posttreatment arteriogram demonstrating nonfilling of both aneurysms. The posterior communicating aneurysm was treated with a single GDC-10 2.5-mm × 4-mm ultrasoft coil, resulting in a CER of 2.4%.
C, One-year follow-up arteriogram reveals obliteration of both aneurysms.
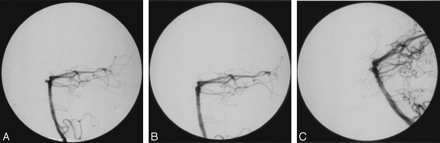
Four recurrences (16%), one large and 3 small (≤1 mm), were identified. The 3 small recurrences were so diminutive that they would not hold additional coil material (Fig 2). The patients with these abnormalities received no further intervention. Recurrence was recognized in 3 of the 7 (43%) anterior communicating aneurysms, and in one of the 3 basilar aneurysms. Two of the 3 recurrent anterior communicating aneurysms had previously ruptured. The recurrent basilar aneurysm had previously ruptured as well. The average CER for unchanged aneurysms was 8.0% (SD, 5.9%). CER values for the recurrent aneurysms were 2.6%, 2.9%, 8.6%, and 21.1% (average, 8.8%; SD, 8.7%). There was not a significant difference between these 2 groups (P = .8203).
Small recurrence, patient 12, a 46-year-old woman with a ruptured 3-mm basilar apex aneurysm.
A, Lateral vertebral arteriogram showing the 3-mm anteriorly directed basilar apex aneurysm.
B, Lateral vertebral arteriogram immediately after treatment with a single GDC-10 2.5-mm × 6-cm ultrasoft coil showing the nonfilling of the aneurysm. The CER was 8.6%.
C, Six-month follow-up lateral vertebral arteriogram reveals a small recurrence at the base of the aneurysm.
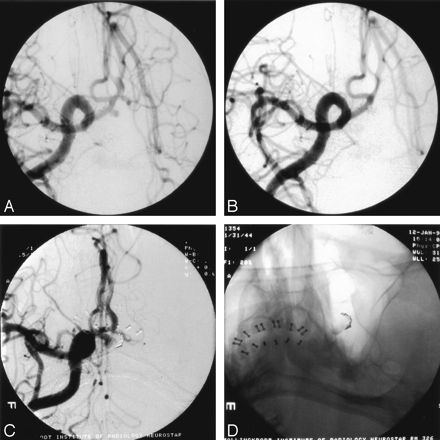
The average CER for all aneurysms was 8.2% (SD, 6.2%; range, 0.6%–21.1%; Table). Only 3 (12%) of the aneurysms had a CER >20%. Two of these remained stable. The other aneurysm (an anterior communicating artery lesion) had a large (6 × 10 mm) recurrence that was demonstrated 2 months after treatment, when the patient returned with a second subarachnoid hemorrhage (Fig 3). This recurrence was obliterated by surgical clipping without sequelae. No other recurrence resulted in new subarachnoid hemorrhage.
Large recurrence, patient 18, a 53-year-old man with a ruptured anterior communicating artery aneurysm.
A, Frontal waters right carotid arteriogram showing a 2.5-mm anterior communicating aneurysm.
B, Immediate posttreatment image reveals obliteration with a single GDC-10 2-mm × 8-mm soft coil. The CER was 21.1%
C, Surveillance arteriogram 2 months later shows coil compaction and a 6 × 10 mm recurrence.
D, Nonsubtracted single oblique waters image shows the compacted single coil at the periphery of the aneurysm as well as stagnant contrast and thrombus within the aneurysm.
Patient and aneurysm characteristics
Discussion
The International Study of Unruptured Intracranial Aneurysms (ISUIA) has prompted considerable discussion about the proper management of small intracranial aneurysms (9, 10). Although the ISUIA data indicate that in the absence of additional previously ruptured aneurysms, small anterior circulation aneurysms only carry a 0.1% per year risk of rupture, many experienced neurosurgeons and endovascular therapists report that most ruptured aneurysms encountered in practice are small (11). Furthermore, it has been suggested that ruptured small aneurysms may cause larger subarachnoid hemorrhages than larger aneurysms (12). Thus, smaller lesions may be more dangerous if they rupture.
Although small aneurysms are frequently encountered, the endovascular treatment of these lesions can be technically demanding. Small size makes for challenging aneurysm catheterization, risk of dome perforation by microcatheters that load and spring forward, and difficulty placing multiple coils. Detachment of coils within small aneurysms is difficult due to the relatively large diameter of current coils with respect to the size of the dome. On occasion, diminutive aneurysm size only permits treatment with a solitary embolization coil owing to a combination of these factors.
Aneurysm recurrence is a known disadvantage of endovascular coil embolization when compared with surgical clipping. Recurrence is often due to coil compaction (13–15). CER may be a useful measurement to estimate the likelihood of coil compaction resulting in aneurysm recurrence. A CER of 20%–33% is optimal (4–8). Although filling an aneurysm with as much coil material as possible likely reduces the risk of lesion recurrence, the efficacy of the CER in predicting the stability of small single-coil-embolized aneurysms has not yet been determined. This study aimed to determine how much of a small aneurysm’s volume is filled with embolic material when treated with a single coil and to establish the efficacy of calculating packing attenuation in these lesions.
In this series, only 3 (12%) of the single-coil-treated aneurysms had a CER >20%. In fact, the average CER of 8.2% for the study aneurysms was less than half of the reported minimum optimal CER of 20%; however, the low packing attenuation of the aneurysms was not predictive of future recurrence. Only 3 of the 22 aneurysms with CER <20% recurred. The fourth recurrence was found in one of the 2 lesions that had a CER of >20%. The overall recurrence rate in the series (16%) was in keeping with the recurrence rate of coil-embolized intracranial aneurysms of all sizes, which was estimated to range from 17.2% to 33.6% in 2 large recently published series (16, 17). Placement of a single coil into a small aneurysm may offer protection from future recurrence, and therefore rupture, despite the modest average packing attenuation of these diminutive lesions.
Aneurysm recurrence is a multifactoral problem. The results of this series suggest that factors other than the CER may be more important in predicting stability of small aneurysms that can be treated with only a single detachable coil. Rupture status may be significant. In this small series, 3 of the 4 recurrences occurred in previously ruptured aneurysms. Potential reasons for this finding may include a tendency to use coils of slightly smaller diameter when treating an acutely ruptured lesion. Perhaps aneurysmal thrombus is more common in recently ruptured aneurysms, resulting in coil compaction when thrombus dissipates. Location may be an important predictor, because recurrence was recognized in 3 of the 7 (43%) anterior communicating aneurysms. Although not evaluated in this series, geometric features such as dome-to-neck ratio may also prove to be helpful in predicting stability of this aneurysm subset (18–20).
The relationship between CER and aneurysm stability in these small aneurysms illustrates the complex nature of the two related phenomena of coil compaction and aneurysm recurrence. The attenuation of coil packing is clearly not the only factor involved. One possible explanation is that adequate flow diversion at the aneurysm neck may be achieved with a single coil in these small aneurysms, allowing thrombosis and scar formation. Bavinzski et al examined 15 aneurysms in patients who died between 30 and 40 days after treatment with GDCs (21). In small aneurysms (diameter <12 mm), the aneurysm neck was covered by a fibrinous membrane as early as 6 days and no later than 10 days after placement of coils. Only one of 5 large aneurysms in their series, studied between 9 and 22 days after GDC, developed a membrane across the neck.
Though small and retrospective, this series suggests that most small, single-coil-embolized aneurysms have a relatively low CER but that they are as well protected as larger multicoil-embolized lesions. Future study of this unique but relatively common lesion may yield additional information that will help dictate safe and effective treatment.
Conclusion
Small intracranial aneurysms treated with a single detachable coil demonstrated satisfactory stability despite having a low average packing attenuation. The CER was not predictive of recurrence in this subset of small cerebral aneurysms. The calculation of CER to help guide treatment decisions is not necessary in patients with small aneurysms.
Footnotes
Presented at the 42nd Annual Meeting of the American Society of Neuroradiology, Seattle, Washington, June 7–11, 2004.
References
- Received December 15, 2004.
- Accepted after revision February 17, 2005.
- Copyright © American Society of Neuroradiology