Abstract
SUMMARY: We report an unusual etiology for a thromboembolic complication. Occlusion of the middle cerebral artery occurred before embolization of an intracranial aneurysm. Attempts to recanalize the artery failed by using both fibrinolytics and IIb/IIIa inhibitors but succeeded with mechanical thrombectomy with a microsnare. Pathologic analysis of the thrombus showed numerous synthetic fibers that were determined to have originated from unsealed gauzes that were used during the procedure.
Thromboembolic events are the most frequent complications of endovascular treatment of intracranial aneurysms, seen on angiography in 9%.1 These may be due to inadequate flushing of the guiding catheter or thrombus extension from the aneurysm. Embolic events are more frequently recognized when diffusion-weighted MR imaging is performed following treatment with one series reporting an incidence of 61%.2 Most of these events remain clinically silent and are not visible on angiography. The exact etiology for these lesions is usually unknown. We report an unusual cause of thromboembolic occlusion of the middle cerebral artery due to inadvertent injection of synthetic fibers.
Case Report
A 43-year-old woman with a recent episode of subarachnoid hemorrhage was referred for endovascular treatment of 2 intracranial aneurysms located at the right carotid bifurcation and left posterior communicating artery.
Embolization was achieved by using standard anticoagulation protocol with a bolus of 50 IU/kg of heparin followed by a continuous infusion of 25 IU/kg/h. Continuous flushing of the guiding catheter (Envoy 6F; Cordis, Miami Lakes, Fla) and microcatheter (Prowler 14, Cordis) with saline was achieved throughout the procedure. Both aneurysms were intended to be treated in the same session. After uneventful embolization of the right carotid bifurcation aneurysm, the 6F guiding catheter was displaced from the right to the left internal carotid artery. Injection of the left internal carotid artery was initially normal but showed an abrupt occlusion of the left middle cerebral artery after insertion of the microcatheter (Fig 1). Embolization of the posterior communicating artery aneurysm was rapidly achieved followed by intra-arterial fibrinolysis with 900,000 IU of urokinase. Fibrinolysis remained unsuccessful so that intra-arterial abciximab (ReoPro, Centocor, Malvern, Pa) was progressively added, with a total injected bolus of 8 mL. A slight reduction of the thrombus was found, but the middle cerebral artery remained partially occluded. Mechanical thrombectomy was therefore attempted by using a 2-mm microsnare (Microvena, EV3, Irvine, Calif). The thrombus was caught at the third attempt and could be removed, allowing complete recanalization of the middle cerebral artery (Figs 2 and 3). On wakening, the patient had no motor deficit but a mild aphasia that progressively resolved within 6 months.
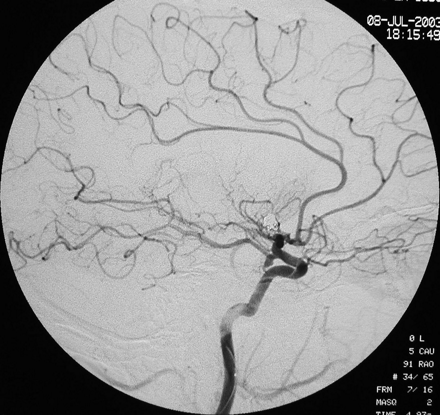
Lateral projection of the left internal carotid artery after insertion of the microcatheter showing an acute occlusion of the middle cerebral artery.
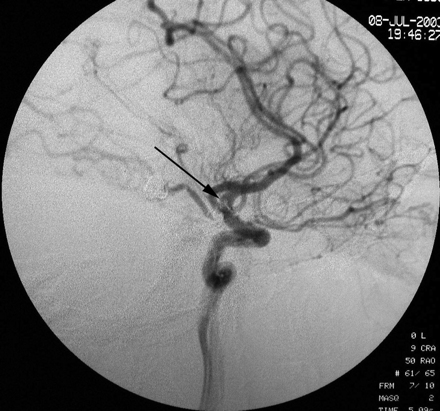
Immediate angiographic control after removal of the thrombus with a goose-neck microsnare. The thrombus can be seen at the level of the carotid siphon (arrow).
Removal of the thrombus that is caught in the goose-neck microsnare.
The thrombus was sent to pathology for analysis. Numerous synthetic fibers were found within the thrombus. Confirmation of foreign bodies was achieved with polarized light examination (Fig 4). All possible sources for spillage of synthetic fibers were checked. Unsealed gauzes were found within the angiography vascular set (Cardinal Health, McGaw Park, Ill). These gauzes consisted of a double-layer gauze containing cotton-like material. The layer was disrupted on one side, allowing spontaneous spillage of the fibers (Fig 5). These fibers were found to be widespread in the entire vascular set. Microscopic examination and chromatographic analysis confirmed that fibers found in the gauzes, vascular set, and thrombus were similar. This led to withdrawal from the vascular set of these unsealed gauzes by the manufacturer.
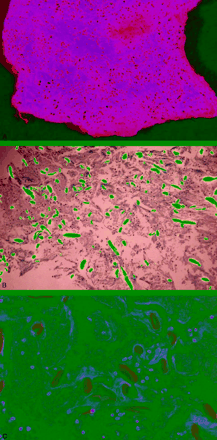
A, Macroscopic view of the thrombus showing fibers inside the platelets aggregates.
B, Polarized light showing the presence of foreign bodies inside the thrombus.
C, Regular oval-shaped fibers suggesting a synthetic origin.
Unsealed gauze furnished in the vascular set. Spontaneous leakage of unattached fibers may be seen after minimal handling of the gauze.
Discussion
Thromboembolic events may occur during intracranial endovascular procedures despite adequate anticoagulation. Intra-arterial injection of fibrinolytic drugs and/or IIb/IIIa inhibitors may be achieved, allowing a high recanalization rate when the thrombus is fresh and unorganized.3,4 In our patient, the thromboembolic occlusion was initially presumed to be platelet derived, but inefficiency of the fibrinolytic agents led us to attempt a mechanical thrombectomy. Thrombectomy may be achieved by using either thromboaspiration as reported in the basilar artery5 or a dedicated device.6,7 In our patient, the goose-neck microsnare allowed rapid recanalization without thrombus fragmentation and capture of the thrombus so that further histologic analysis could be achieved and the synthetic fibers found.
Intravascular embolism related to foreign material has rarely been described. Fragmentation of the hydrophilic coating from microcatheters has been reported.8 Leakage of synthetic fibers due to fragmentation of vascular dacron prostheses9 have also been reported.
In our patient, the embolus was made of numerous synthetic fibers that migrated and blocked the middle cerebral artery. It may be argued that the fibers were secondarily incorporated into a pre-existing thrombus originating from the guiding catheter. The fibers, however, were numerous and evenly distributed throughout the entire thrombus. This is more likely to occur if the thrombus formed around the fibers and not if the fibers were secondarily mechanically incorporated into a pre-existing thrombus. Moreover, the number and volume of these thrombogenic fibers was sufficient to occlude an artery. Besides, if the middle cerebral artery would have been primarily occluded by a pre-existing thrombus, the flow would have been reduced making secondary migration and incorporation of fibers in the thrombus less likely. These fibers originated from unsealed gauzes, as confirmed by spectrometric analysis. These fibers were disseminated within the vascular set. It may therefore be presumed that some fibers were mixed in the saline and contrast and were directly injected in the guiding catheter. This kind of embolic complication may be underestimated, because histologic analysis of a thrombus is usually not achieved as this requires mechanical retrieval. Individual similar fibers may, however, occasionally be observed in arterial lumen of histologic specimens of tumors and arteriovenous malformations operated on after embolization. Silent thromboembolic events are frequently diagnosed on MR diffusion sequences after cerebral or cardiac angiography with an incidence ≤62%.2 These lesions may be attributed to inadvertent injection of micro bubbles of air- or platelet-derived thrombi. The incidence of such lesions can be significantly reduced by using a filter placed at the proximal part of the catheter, as shown in a prospective randomized study.10 This kind of filter may also be used to prevent inadvertent injection of foreign material such as synthetic fibers.
Simultaneously, unsealed gauzes must be withdrawn, as well as gauzes that are likely to fragment and deliver synthetic fibers. Such kinds of fragmentation may be induced by maneuvers of catheterization such as the use of gauzes to manipulate hydrophilic guidewires. Torquing of hydrophilic guidewires may be facilitated by gauze, though this promotes drying of the hydrophilic coating with subsequent sticking of the gauze to the guidewire and detachment of fibers when removing the gauze.
Acknowledgments
Special thanks to Dr. Hubert L’Hôpital for his expert review of the manuscript.
References
- Received December 28, 2004.
- Accepted after revision February 17, 2005.
- Copyright © American Society of Neuroradiology