Abstract
BACKROUND AND PURPOSE: Our aim was to evaluate the safety and clinical utility of a fluoroscopically guided anterolateral oblique approach technique for outpatient diagnostic and therapeutic selective cervical nerve root blockade (SCNRB).
MATERIALS AND METHODS: During a 13-year period (1994 through February 2007), 4612 patients underwent fluoroscopically guided diagnostic and/or therapeutic extraforaminal SCNRB by using an anterior oblique approach at affiliated outpatient imaging centers. Each procedure was performed by 1 of 6 procedural radiologists, all highly experienced in and actively performing spinal injections on a full-time basis in clinical practice. All of the proceduralists were thoroughly experienced with lumbar injections before endeavoring to perform SCNRBs. Nonionic contrast was injected in nearly all patients (except isolated patients with contrast allergy), and a minimum of 2 projection filming procedures were performed to document the accuracy of needle placement and contrast dispersal before the injection of therapeutic substances. All clinically significant complications beyond skin discoloration and temporary exacerbation of symptoms were recorded.
RESULTS: There were no serious neurologic complications, such as stroke, spinal cord insult, or permanent nerve root deficit. One life-threatening anaphylactic reaction occurred and was attributed to the injected materials and not the specific procedure itself. Another patient had a 3- to 4-minute grand mal seizure, from which he fully recovered within 30 minutes. There were no infections.
CONCLUSION: The technique we describe for fluoroscopically guided SCNRB is a useful and safe outpatient procedure when performed by skilled and experienced proceduralists.
Transforaminal selective cervical nerve root blockade (SCNRB) is used both as a diagnostic and therapeutic procedure in patients with cervical radiculopathy. There is general agreement and support that image guidance is required for SCNRB to be performed safely and accurately.1–9 CT,1,2,4,5 CT fluoroscopy,7 and fluoroscopy3,6,8,9 are all used and advocated for image guidance during performance of this procedure. Meticulous attention to needle placement with fluoroscopic guidance has proved that neurologic complications can be avoided.9 An interlaminar catheter technique for SCNRB by using fluoroscopy has also been described10,11 and challenged12 as an alternative to either the lateral or anterolateral approach. Devastating neurologic complications, including cerebral and spinal cord infarction, have been described with SCNRB,13-24 resulting in some questioning the safety and appropriateness of this procedure in clinical practice.25–29 We reviewed our series of 4612 cases of either 1- or 2-level (sequential) SCNRB using an antero-oblique approach performed for 13 years by 6 different procedural radiologists and found no serious irreversible neurologic complications. We describe our technique and discuss why we believe it allows us to perform this procedure safely and accurately.
Materials and Methods
In the years 1994 through February 2007, 4612 patients (aged 17–83 years) underwent outpatient percutaneous SCNRB at 1 of 8 outpatient imaging centers in the Minneapolis-St Paul metropolitan area (Center for Diagnostic Imaging, St Louis Park, Minn). Each procedure was performed by 1 of 6 highly experienced procedural radiologists, engaged in the full-time practice of spine injection.
Procedures were performed to either investigate and diagnose the origins of cervical radiculopathy or therapeutically intervene in such pain or both. All patients were referred for injection by clinicians having no financial relationship whatsoever with either the imaging/diagnostic centers or the radiologists performing the procedure. Specialties referring patients for SCNRB included orthopedic spine surgery (most frequently), neurosurgery, neurology, physiatry, anesthesia-pain management, internal medicine, and family practice. The C6 and C7 roots were the most frequently studied levels. C5, C4, and C8 were blocked with considerably lower frequency.
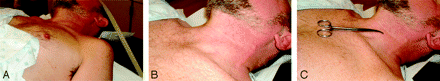
Procedures were performed in a dedicated special-procedures suite equipped with a highly specialized table with multidirectional tabletop movement capability, including head-up and head-down tilt, elevation change, side movement, side roll, and high-resolution multidirectional C-arm fluoroscopy with magnification and filming capability. After giving informed consent, the patient was placed supine on the table with the neck extended and chin deviated approximately 30° away from the side where the injection was to be performed (Fig 1). To prevent the patient from moving suddenly, we then placed 1¼-inch paper adhesive tape across the forehead and secured it to the tabletop on both sides. Initially, fluoroscopy was used to determine the optimal fluoroscopic axis for the needle approach and to mark the skin at the site for needle placement, by using the butt end of a ballpoint pen to place a circular dent in the skin. The target for final needle-tip placement was the lower lateral inferior aspect of the nerve root canal peripheral to the neural foramen at the desired cervical spinal segment. Thereafter, the skin was thoroughly sterilized with iodine solution, which was allowed to dry for approximately 2 minutes, after which it was rinsed with alcohol and a fenestrated plastic adhesive sterile drape was applied.
Patient positioning for SCNRB on the left side. A, The chin is rotated approximately 30° to the right, away from the side to be studied. To prevent sudden movements during needle placement/manipulation, we used 1¼-inch paper adhesive tape. B, Note how the patient's neck is rotated and slightly extended. C, A metal clamp is used to help identify the optimal axis of fluoroscopy.
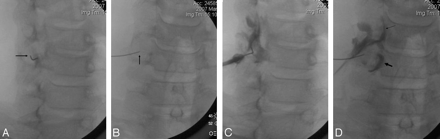
A 25-gauge spinal needle (with a sharp point) was then carefully advanced through the skin (no local anesthesia), using a slight caudal-to-cephalad approach in most cases, depending upon body habitus, along the axis of fluoroscopy but without live fluoroscopy (which has only rarely been used) toward the target anatomy. Intermittent fluoroscopy, by using the low-dose mode for 0.25–1.0 seconds on average, was then used to check the needle position and alignment until the target anatomy was reached. The needle was then advanced until either the cervical spine was contacted or radicular pain was provoked (Fig 2A, -B). If radicular pain was provoked during needle placement, the needle was carefully withdrawn a few millimeters, redirected by using bevel rotation, and advanced until the bony spine was contacted. Thereafter, a test injection of 1–2 mL of full-strength contrast (Iohexol [Omnipaque], GE Healthcare, Princeton, NJ) (240 mg/mL) was performed (after connecting the contrast syringe with a 24-inch connecting tubing to the needle) during live fluoroscopic observation to assess needle position relative to the desired target anatomy (Fig 2C, -D). In each case, the needle tip was placed against bone, deep to the nerve, in the posteroinferior lateral aspect of the neural foramen to ensure depth control and stable positioning of the needle. If the needle position was judged satisfactory, proximal and distal nerve root opacification was observed (Figs 2C, -D, 3–5).
Sequential fluoroscopic images demonstrate optimal needle placement (A and B) and contrast injection (C and D). A, Approximately 45° oblique projection, with slight caudal-to-cephalad orientation of the fluoroscopy axis, is centered on the lower lateral aspect of right C5–6 foramen. Needle tip (arrow) is in contact with the posterior wall of nerve root canal. B, AP image shows the needle tip (arrow) optimally positioned in the lower lateral aspect of right C5–6 neural foramen, with the needle tip contacting bone. C and D, Approximately 45° oblique (C) and AP (D) images obtained after injection of approximately 1.5 mL of contrast. The right C5 nerve root is outlined by contrast. Epidural reflux at C5 (small arrow) and C6 (larger arrow) is clearly demonstrated in D. This case represents an optimal degree of opacification for a therapeutic blockade, in which epidural reflux is desired.
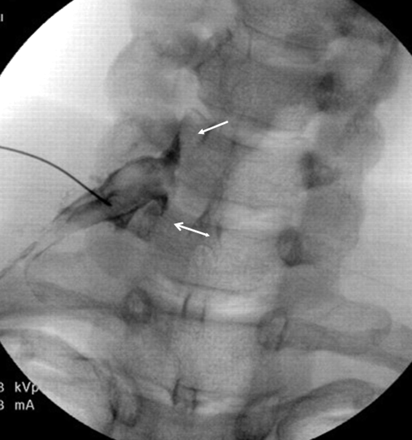
Optimal right C7 nerve root opacification before therapeutic injection. AP projection shows contrast surrounding the right C7 nerve root and ganglion, with epidural reflux above and below (arrows).
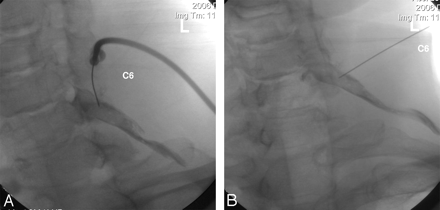
Approximately 45° oblique (A) and AP (B) images revealing mostly peripheral opacification of the left C6 nerve root. Approximately 1.5 mL of contrast was injected before obtaining these images. Such mostly peripheral nerve root opacification is ideal for a diagnostic SCNRB.
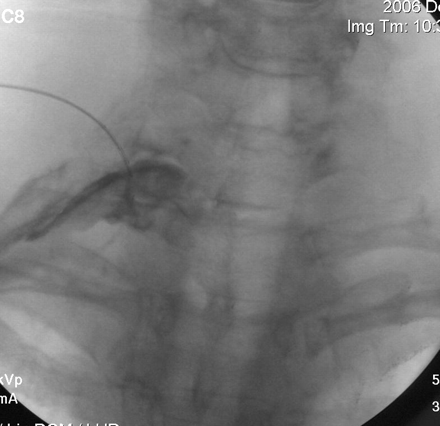
Ideal opacification of the right C8 nerve root before therapeutic injection. AP projection with caudal-to-cephalad fluoroscopy angulation of approximately 30°, intended to be parallel with the C7–T1 disk axis. Note how the needle comes from above; this approach is the only way to safely and successfully access the C8 nerve root and avoid the pulmonary apex. The nerve root is opacified proximally and distally with approximately 1 mL of contrast.
Anteroposterior (AP) and oblique films were then obtained by using most often the 6-inch intensifier mode, and therapeutic injection was made taking extreme care to not move the needle during the exchange of the contrast-filled connecting tubing and the syringe containing therapeutic substances. If less than optimal nerve root filling and/or vascular opacification was observed (Fig 6), fine-needle manipulations were performed to reposition the needle tip, followed by repeat contrast injections until optimal needle position and nerve root opacification (without vascular filling) was obtained. Fluoroscopic spot films in the AP and oblique projection were then obtained in every case to document contrast dispersal. After satisfactory/optimal needle positioning, contrast injection, and filming, patients were questioned about their experience (if this was a familiar sensation in location and character) during injection. After filming and brief questioning of the patient, we injected a therapeutic mixture of lidocaine, 2%, mixed with either betamethasone acetate suspension (Celestone Soluspan; Schering, Kenilworth, NJ) or generic/formulated sodium phosphate (40 mg/mL) or methylprednisolone sodium phosphate or acetate (Depo-Medrol; Pharmacia, Kalamazoo, Mich) in a 1:1–3:1 mixture in a total volume of 1.0–1.6 mL. Care was taken to inject no more than 1.6 mL and ideally tailor the volume and mix of therapeutic injectant on the basis of the prior contrast dispersal pattern, the amount of contrast injected, and the purpose of the procedure that was being performed, primarily diagnostic versus therapeutic. After injection of therapeutic substances, the needle was removed and light pressure was applied to the injection site for approximately 30–60 seconds.
Venous opacification observed during contrast injection into the lower lateral aspect of the right C6–7 nerve root canal, along the C7 nerve root. A, AP projection revealing prominent venous opacification (arrows) below the site of injection. Such venous opacification would not be visible on thin-sectioned CT, being out of the plane of data acquisition. B, After needle manipulation, repeat injection shows improved opacification of the C7 nerve sheath (curved arrows), but new venous filling above the nerve root (arrow).
Patients were then removed from the fluoroscopy suite and taken to a waiting room where they could be continuously observed and monitored for therapeutic response during the next 20–45 minutes. Patients were asked to rate their response to injection 20–30 minutes postprocedure and describe a percentage estimate regarding the degree of pain relief obtained (0%–100%). After obtaining their response to injection, we checked their status, after which they were discharged, provided that there were no complications or negative side effects requiring further observation. All patients were required to either bring an alternative driver with them or be transported. In the most recent 3 years, since we have had a certified ambulatory surgical center (ASC) available to us within 1 of our centers, approximately 5% of SCNRBs have been performed with light conscious sedation, most often in patients demanding sedation because of a negative prior experience elsewhere. Other procedures electively performed in the ASC included patients considered to be at higher than usual risk due to various medical circumstances. Each patient was carefully observed and questioned about any problems before discharge. Patients were instructed to call us first during the next week in the event of any questions, swelling, local redness, increased pain, fever, or problem that they experienced that might relate to the procedure.
Results
There were no permanent nerve root, spinal cord, brain stem, or cerebellar/cerebral infarcts/insults in the series. There were also no infectious complications. A 45-year old male patient had a grand mal seizure lasting 3–4 minutes within 10 seconds of therapeutic injection of 1.5 mL of a mixture of 2 parts lidocaine 2% and 1 part generic formulated betamethasone along a C6 nerve root. He recovered completely within 30 minutes without requiring any additional medications beyond nasal oxygen and IV saline. One female patient had a life-threatening generalized anaphylactic reaction (from which she recovered fully) to injected materials (generic betamethasone acetate) minutes after completion of the SCNRB. We are still uncertain whether her reaction was to the contrast or the formulated steroid solution, which we completely stopped using after that event. Approximately 5% of patients (usually those who did not respond to the injection with pain relief) described exacerbation of clinical pain for up to 10 days postprocedure, though all of these patients’ pain ultimately returned to preprocedure levels. Minor and temporary skin discoloration up to 3 cm around the site of injection was encountered in a small number of patients (no exact count). In each patient, this resolved within 14 days.
Discussion
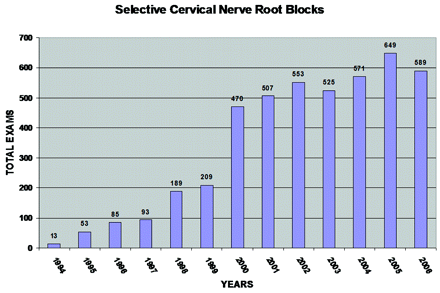
The technique we describe evolved from considerable experimentation regarding how to perform SCNRB safely and reproducibly as requests for this procedure grew. There was a paucity of technique literature (especially radiologic) in 1993 when referrals for SCNRB began to increase. The anterolateral approach using a sharp pointed 25-gauge needle, which we described and was introduced by author S.R.P. in early 1993, was thereafter used by the first 2 investigators (S.R.P, K.P.S.) for several procedures each, and was agreed upon as the safest and most reliable technique, and has been used by all members of our local practice since. We have made only a few minor refinements in this technique since the original implementation, and this has become one of the more frequently performed studies in our practice (Fig 7). Minor individual technical variations are exercised by each proceduralist to address special and unique circumstances encountered.
Graph shows the volume of SCNRBs performed in 1994 through 2006.
High-resolution C-arm fluoroscopy best facilitates SCNRB, though CT and/or CT fluoroscopy have been described and advocated by others.1,2,4,5,7 A series of 1036 cases (without any serious complications) of fluoroscopically guided SCNRB using a lateral approach support our opinions regarding fluoroscopy and the safety of this procedure.9 Careful positioning of the patient and determination of fluoroscopic axis before sterile preparation and needle introduction circumvent the need for live fluoroscopy of the proceduralist's hands during needle manipulation and placement (Figs 1 and 2A, -B). We carefully advance the needle incrementally with 0.5- to 1-second fluoroscopy checks (hands removed from the field) until we either encounter the bony spine or the patient feels pain, after which a small amount of contrast is injected during magnified fluoroscopic observation (Fig 2). If we have accomplished optimal nerve sheath opacification (Figs 2C, -D, 3–6), after injecting up to a maximum of 1.5 mL of contrast at that site, we obtain images in AP and oblique projections. Thereafter, therapeutic injection is made through the needle after disconnecting the contrast syringe and connecting tubing. Often, additional needle manipulations are required after initial introduction until the desired nerve root is opacified and there is no observed vascular opacification. We do not inject steroid and anesthetic until we achieve optimal nerve root opacification and have ruled out any significant (especially arterial) filling with contrast. This can be challenging in cases of foraminal and/or central spinal canal (especially multilevel) stenosis, in which venous collaterals are often encountered. We have found that needle placement into the lower lateral aspect of the desired neural canal most often leads to a successful study. A typical procedure takes approximately 15 minutes from initial physician-patient contact in the procedural suite and less than 5 minutes from initial needle introduction to procedure completion. Fluoroscopy time rarely exceeds 1 minute for the entire procedure.
The applications of SCNRB as both a diagnostic and/or therapeutic procedure have increased substantially in recent years, as has literature pertinent to this subject.1–28 In our own practice, referrals for SCNRB have grown rather dramatically from 13 cases in 1994 (Fig 7). Diagnostic blockade is most often performed to evaluate whether clinical radicular pain is related to neural impingement observed on imaging studies and to identify which nerve or nerve roots may be involved in clinical pain generation. When diagnostic blockade is successful and thereafter provides substantial and lasting pain relief, the procedure may be repeated at a later date and may become a purely therapeutic endeavor. Different injection techniques and mixtures of anesthetic and steroid exist; however, all diagnostic injections include local anesthetic, and all therapeutic interventions involve the use of the anti-inflammatory steroids.3,8,9,27,28 In our practice, an initial diagnostic SCNRB would use 1.0–1.6 mL of a mixture of 1 part steroid and 2- to 3-parts lidocaine, whereas a repeat (therapeutic intent) injection for the purpose of pain management might use 1.3–1.6 mL of a mixture of 1 part steroid to 1–2 parts lidocaine.
The technique we describe emphasizes the use of high-resolution fluoroscopy, undiluted nonioninc contrast media, and an anterolateral approach for needle placement. We do not perform needle placement and manipulation under live fluoroscopy; however, magnification fluoroscopy, live observation, and filming are always performed during contrast injection. Digital subtraction angiography is not routinely performed in our practice; however, it is available whenever we have a question about possible arterial opacification. We consider the use of undiluted contrast and live fluoroscopic observation during contrast injection to be crucial. We decline most (but not all) referred cases of SCNRB where there is a history of contrast allergy, especially patients with long-standing diabetes mellitus, generalized poor health and frailty, and advanced age with multilevel advanced degenerative spondylosis. Live observation of the contrast injection under magnified high-resolution fluoroscopy is the only way to confidently rule out arterial opacification.
All of the serious neurologic complications of SCNRB described occurred either during or immediately following the procedure,13–26 and there exist differing theories regarding the pathogenesis of such events. Obviously, direct injection of steroid and/or anesthetic into the spinal cord, a cervical nerve root, or the vertebral or a spinal radicular artery has the potential for permanent and devastating insult. We are uncertain about the seizure etiology in our patient, who fortunately recovered completely. An unintended intra-arterial injection of steroid and anesthetic must be considered; however, we experienced a similar complication in a patient after lumbar injection with the same generic betamethasone mixture. We terminated the use of this mixture after having a number of serious complications and have had no seizures or anaphylactic reactions in more than 20,000 spinal injection procedures since discontinuing the use of that mixture. Such complications can be avoided by a highly skilled and experienced proceduralist using an optimal technique before therapeutic injection (provided that the medicinal substances are safe).9 The separation of anesthetic (first) and steroid solution (second) injections is advocated as a safety measure to decrease/eliminate the risk of stroke.21–23,27 We have always injected a single mixed solution of anesthetic and steroid, except in patients in whom we were unable to use contrast because of allergic history. Our technique of antero-oblique approach and targeting the lateral inferior aspect of the neural canal ensures that the needle tract is lateral to both the carotid and vertebral arteries. We use exclusively skinny (25 gauge) sharp pointed needles because they are easy to direct and small enough to avoid arterial injury in the event of an unintended arterial puncture. Some authors recommend using blunt needles for SCNRB to theoretically avoid arterial injury and/or intra-arterial injection.29 Our experience and results prove that sharp needles are safe, in skilled hands using proper technique.
The size of steroid particles and the tendency for those various particles to clump or aggregate is believed to influence the likelihood of neurologic insult and tissue infarction in the event of an inaccurate injection into either neural tissue or a supplying artery. The fact that we have used either betamethasone sodium phosphate or betamethasone acetate suspension (both with a tendency to aggregate) mixed with lidocaine 2% in more than 75% of our procedures testifies to the safety of our technique. In the other cases, methylprednisolone acetate (MPA) (40 mg/mL) mixed with lidocaine 2% was and is used, also without any serious neurologic insults. MPA is characterized by small particles (mostly smaller than red blood cells) with little tendency to aggregate. We have begun using dexamethasone sodium phosphate mixed with lidocaine for most cases of SCNRB since completion of this study, because recent literature describes this steroid as having the smallest particles and little or no tendency to aggregate. Because there are currently no documented reports of stroke associated with dexamethasone, there may be no need to separate the anesthetic and steroid injections as some advocate. We continue to mix our local anesthetic and steroid solution because we adhere to the previously described technique and are phasing out the use of particulate aggregation–prone steroids for SCNRB. We are prospectively recording all and any SCNRB complications since introducing this different steroid and have had none in the first 500+ cases.
With regard to the clinical utility of SCNRB, some thoughts and observations are in order. The demand for this procedure (both diagnostic and therapeutic) has been generally increasing since we first offered the procedure in circa 1990 (Fig 7). An important fact about this demand in our practice is that every single patient was referred by clinicians (mostly orthopedic spine and neurosurgeons) with no financial relationship with ourselves whatsoever. There have never been any financially motivated referrals in our practice. Every patient was (and continues to be) legitimately referred to us because the clinicians caring for the patient believed that SCNRB would provide useful and necessary diagnostic information and/or therapeutic benefit.
It is our practice that SCNRB should not be performed by anyone who is not thoroughly experienced with fluoroscopic imagery, spinal anatomy, and pathology, and ideally less challenging spinal injections such as lumber procedures. We consider SCNRB to be the most challenging injection that we perform (including cervical and thoracic diskography). All new proceduralists in our practice thoroughly master selective lumbar nerve root blockade (and other lumbar procedures) before performing SCNRB. There is definite potential for serious injury and resultant permanent disability, so this procedure needs to be approached with utmost caution and preliminary mastery of less dangerous studies.
Conclusion
The technique we describe has stood the tests of numbers and time. In skilled and experienced hands, SCNRB is a safe and valuable procedure for both diagnosis and treatment of cervical radicular pain.
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Spine Radiology, February 24, 2007; Chicago, Ill.
References
- Received March 9, 2007.
- Accepted after revision April 12, 2007.
- Copyright © American Society of Neuroradiology