Abstract
BACKGROUND AND PURPOSE: The goal of this study was to prospectively assess the feasibility, safety, and efficacy of balloon disruption of the middle cerebral artery (MCA) by using a deflated balloon catheter combined with an intra-arterial thrombolysis for the treatment of acute ischemic stroke.
MATERIALS AND METHODS: Seven consecutive patients with clinical findings of acute major-vessel stroke met our criteria and underwent balloon disruption of an MCA thrombus with a deflated balloon catheter. The balloon disruption was performed with a low-profile microballoon catheter. The microballoon was inflated in the distal carotid artery and then deflated and advanced just distal to the occlusion site in the MCA. Thereafter, an intra-arterial thrombolysis of the MCA was applied. The maximum time from the onset of symptoms to the start of treatment and maximum dosage of urokinase was 6 hours and 600,000 U. The outcome was classified as good for a modified Rankin Scale (mRS) score of 0 or 1, moderate for a score of 2 or 3, and poor for a score of 4 or 5.
RESULTS: Complete recanalization was achieved in 5 patients and partial recanalization in 3. Three patients recovered to an mRS score of 0 or 1; 3, to scores of 2 or 3; and 1, to a score of 4. No patients died. There was no major intracerebral hemorrhage.
CONCLUSIONS: The penetration of the MCA with a deflated balloon catheter combined with an intra-arterial thrombolysis may be a safe and effective treatment for acute ischemic stroke.
An intravenous thrombolysis using recombinant tissue plasminogen activator (rtPA) has been shown to be an effective treatment for the acute occlusion of the middle cerebral artery (MCA).1 However, the death or dependency rate of patients treated with intravenous rtPA beyond 3 hours after the onset was significantly higher than that of those treated within 3 hours, according to 3 clinical trials and 1 prospective observation.1–4 The limitation of the intravenous approach has led to an evaluation of local intra-arterial thrombolysis (LIT) because of its ability to perform thrombolysis within a 6-hour therapeutic window.5–8 Other benefits of LIT are salvage therapy for intravenous rtPA poor responders and easy evaluation by angiography of the degree of recanalization after treatment.
There are several predicators for LIT, including time to treatment, National Institutes of Health Stroke Scale (NIHSS) scores, and patient age. The most important parameter for the LIT technique itself is to achieve an immediate flow restoration, regardless of the treatment method used. Various techniques have been reported for LIT, including pulse-spray infusion and mechanical disruption by the use of a microcatheter, guidewire, microballoon catheter, or a snare wire.9–16 Direct percutaneous transluminal angioplasty (PTA) using a microballoon catheter is one of the most useful techniques to improve the recanalization rate. However, direct PTA has a potential risk of vessel rupture and injury by perforating arteries. To prevent these risks, we tried disruption of the MCA thrombus with a deflated balloon, which is a simple modified procedure of PTA. To our knowledge, there have been no clinical applications of this new technique.
The purpose of this preliminary study was to evaluate the feasibility, safety, and efficacy of MCA thrombus disruption with a deflated balloon catheter combined with intra-arterial thrombolysis for the treatment of acute ischemic stroke.
Materials and Methods
From December 2003 to June 2005, 16 consecutive patients with acute stroke secondary to cardiogenic emboli underwent emergency angiography. Inclusion criteria for emergency angiography were as follows: 1) clinical diagnosis of acute major-vessel stroke established by a neurosurgeon, 2) presence of an acute hypoattenuated parenchymal lesion on CT or effacement of the cerebral sulci in less than one third of the MCA territory or suspected stroke region, 3) initial NIHSS score of at least 4 points, 4) expected interval from symptom onset to intervention within 6 hours, and 5) patient younger than 80 years of age. Exclusion criteria for emergency angiography were as follows: 1) presence of a high-attenuation lesion on CT, consistent with a hemorrhage of any degree or location, 2) critical general condition, and 3) recent trauma or surgery. Seven patients who were angiographically documented with MCA occlusion and who underwent balloon disruption with a deflated balloon catheter were evaluated for this study. Embolism was located in various major vessels in the study population, including the internal carotid artery (n = 4), the posterior circulation (n = 3), and the M3 portion of the MCA (n = 2). Inclusion criteria for the balloon disruption were the presence of MCA occlusion in the M1 or M2 portion that correlated with the neurologic deficit.
CT angiography, CT perfusion study, MR imaging with diffusion-weighted imaging and perfusion study, or brain single-photon emission CT study were not performed, to minimize the elapsed time to admission for recanalization therapy.
Angiograms were analyzed, and the locations of the occlusion and cerebral perfusion were evaluated by a neuroradiologist who was blinded to the clinical data. The location of the occlusion site in the MCA was classified as proximal MCA; M1 trunk occlusion at or proximal to the lenticulostriate arteries (LSAs), distal MCA; M1 trunk occlusion distal to the LSAs and M2; and division occlusion beyond the bifurcation of M1. The cerebral perfusion before the procedure and the recanalization of the target vessel after it were assessed on the control angiograms obtained just before and after the procedure, according to Thrombolysis in Myocardial Infarction (TIMI) grades,19 as follows: no perfusion, TIMI grade 0; minimal perfusion, 1; partial perfusion, 2; and complete perfusion, 3.
Endovascular Technique
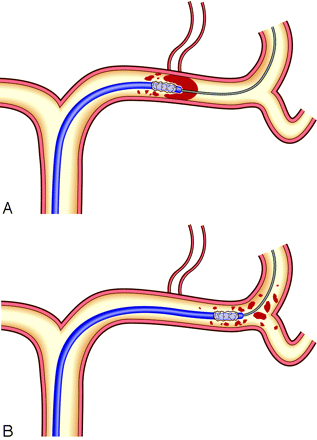
Selective intra-arterial digital subtraction angiography was performed on a high-resolution angiography system (Digitex α plus, Shimadzu, Ibaraki, Japan) with a matrix of 1024 × 1024 pixels. A 6F guiding catheter was placed in the internal cerebral artery (ICA). Through the catheter, a double-lumen microballoon (Gateway PTA balloon catheter, Boston Scientific, Fremont, Calif) with a balloon diameter of 2.0 or 2.5 mm was advanced to the distal ICA. Next, a microballoon was inflated with minimal hand pressure and then deflated and navigated into the occluded MCA along a microguidewire. Because the diameter of ICA was much larger than the microballoon profile, the risk of vessel rupture with balloon inflation was minimal. The penetration and fragmentation of the thrombus were achieved by gently moving forward the deflated microballoon beyond the thrombus (Fig 1). Balloon angioplasty was not performed, to avoid rupturing the vessel walls and perforating arteries. When the embolus was moved to the distal site within M2, this procedure was repeated at the new occlusion site. When it was moved to beyond M3, an injection of the urokinase solution was performed via balloon catheter that was positioned in M2 or M3. The initial dose of urokinase was 120,000 U diluted in 10 mL of saline solution and infused over 5 minutes. The total dose was to be limited to 600,000 U. Thrombolysis was terminated if 1) complete perfusion was re-established by the guiding catheter injection, 2) time expired on the therapeutic window (6 hours since symptom onset), or 3) revascularization was not achieved after a maximum dose of urokinase had been administered.
Disruption of the MCA thrombus by a deflated microballoon catheter; the catheter navigates into the thrombus in the MCA along the guidewire (A). When it is advanced, the thrombus is fragmented (B).
A control CT scan was routinely obtained within the first 24 hours after the procedure to evaluate the hemorrhage and infarction. The outcome was assessed by neurosurgeons, with a clinical examination 3 months after the procedure, by using the modified Rankin Scale (mRS). mRS scores of 0 or 1 were defined as a good outcome; 2 or 3, as moderate; and 4 or 5, as poor.
Results
The recanalization of an occluded MCA by using a deflated microballoon was performed on 7 patients. Their mean age was 69.1 years (range, 48–77 years). The median NIHSS score on admission was 22 and ranged from 17 to 27 (Table). The mean delay from the symptom onset of cerebral ischemia to the procedure was 3.44 hours and ranged from 2.1 to 5.0 hours. The occlusion sites of the MCA were the proximal M1 segment, the distal M1 segment, and the M2 segment in 4, 1, and 2 patients, respectively. Cerebral perfusion before the treatment was TIMI 0 in all 7. After penetration of the MCA by a microballoon, an average of 240,000 U (range, 100,000–420,000 U) of urokinase was administered to provide thrombolysis of the distal emboli.
Clinical and angiographic characteristics and procedural results
The median NIHSS score 7 to 30 days after treatment was 8 and ranged from 0 to 15. Three months after the stroke, 3 patients (43%) had recovered to an mRS score of 0 or 1 (good outcome) and another 3 (43%), to a score of 2 or 3 (fair outcome). The outcome was poor (mRS score of 4 or 5) in 1 patient (14%). No patients died. In a 72-year-old woman (case 6) with poor clinical outcome, the procedure was started 5 hours after the stroke onset and the therapeutic time window expired before sufficient recanalization was attained.
A complete recanalization (TIMI grade 3) was achieved in 5 patients (71%), and a partial recanalization (TIMI grade 2), in 2 (29%). On a CT scan obtained within the first 24 hours after the procedure, there were no patients with intracranial hemorrhage, including minor hemorrhage. A low-attenuation area was demonstrated only in the basal ganglia in 1 patient, it was only minimal in the cerebral cortex excluding the primary motor area in 3 patients, and there was no cerebral low-attenuation area in 3 patients.
Representative Cases
Case 2.
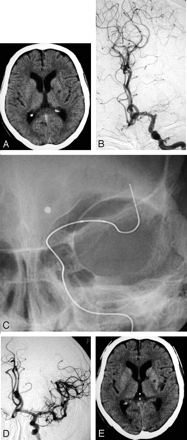
A 76-year-old right-handed man with atrial fibrillation presented with a sudden onset of right hemiplegia and motor aphasia. A CT scan on admission revealed no apparent early finding of low density area (LDA), without small lacunar infarction in the left basal ganglia (Fig 2A). An emergency angiography was performed 4.2 hours after the onset of symptoms and diagnosed an occlusion of the proximal portion of the left MCA (Fig 2B). A thrombus disruption of the left MCA, by using a deflated 2.5-mm Gateway balloon catheter, was performed (Fig 2C). After thrombus disruption of the M1 portion of the left MCA, 120,000 U of urokinase was infused via a Gateway balloon catheter. Just after the treatment, hemiplegia and aphasia were well recovered, and angiography demonstrated complete recanalization, including the LSAs (Fig 2D). A follow-up CT scan obtained 24 hours later revealed no apparent cerebral low-attenuation area, without previous lacunar infarction in the left basal ganglia.
A 76-year-old man (case 2).
A, A CT on admission shows no LDA, without small lacunar infarction in the left basal ganglia. B, A preprocedural angiogram shows an occlusion of the left MCA trunk. C, A deflated microballoon is gently advanced to the embolus. D, An angiogram obtained immediately after the treatment shows complete recanalization. E, On the CT scan obtained 24 hours after treatment, there is no change compared with the initial CT on admission.
Case 7.
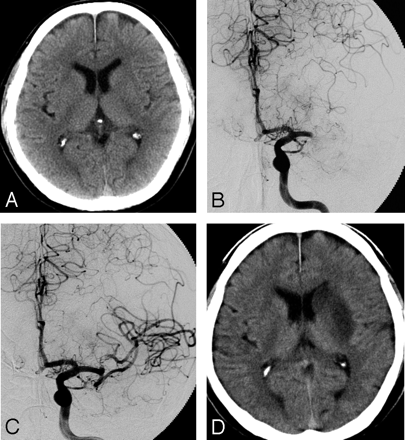
A 63-year-old right-handed man with atrial fibrillation presented with a sudden onset of drowsiness, right hemiplegia, and motor aphasia. A CT scan revealed no abnormality (Fig 3A). An emergency angiography was performed 2.1 hours after the onset of symptoms and diagnosed an occlusion of the proximal portion of the left MCA (Fig 3B). A thrombus disruption of the left MCA, by using a deflated 2.5-mm Gateway balloon catheter, was performed with 420,000 U of urokinase. Just after the treatment, hemiplegia and aphasia were well recovered, and angiography demonstrated complete recanalization, including the LSAs (Fig 3C). A follow-up CT scan performed 24 hours later revealed no LDA in the cerebral cortex, though the infarction in the left caudate head and putamen could not be avoided. Consciousness, right hemiplegia, and aphasia were well improved.
A 63-year-old man (case 7).
A, A CT on admission shows no LDA. B, A preprocedural angiogram shows an occlusion of the left MCA trunk. C, An angiogram obtained immediately after the treatment shows complete recanalization. D, On the CT obtained 24 hours after the treatment, LDA was demonstrated in the left basal ganglia.
Discussion
Intra-arterial thrombolysis and intravenous rtPA have been shown to be effective for the treatment of patients with acute stroke resulting from an occlusion of the MCA.1,6–8,18
One of the most important and immediate parameters for assessing treatment success of the recanalization therapy of the MCA is to evaluate the achieved flow restoration. Recanalization rates with LIT are superior to those for intravenous rtPA for major cerebrovascular occlusions. Recanalization rates for major cerebrovascular occlusions average 70% for LIT, compared with 34% for intravenous rtPA.19 However, recanalization is unsuccessful in approximately 30% of patients treated with LIT alone. This study demonstrated that the present technique resulted in an excellent recanalization rate, with all 7 patients achieving partial or complete recanalization. This contrasts with other studies of MCA recanalization with LIT and intravenous thrombolytic agents, in which recanalization rates ranged from 42% to 100%6,20–25 and with studies of aggressive mechanical embolus disruption series with microguidewire, microcatheter, snare wire, or direct balloon angioplasty, in which partial or complete recanalization rates were from 50% to 100%.9–16
Sorimachi et al14 reported good technical and clinical outcomes with the mechanical embolus disruption, by using a j-shaped guidewire and microcatheter in the carotid territory. Their partial or complete recanalization rate was 100% in patients with MCA occlusion and 91% in those with ICA occlusion. However, iatrogenic perforation by their method was demonstrated in 2 of 23 patients. Direct PTA is now accepted as the standard mechanical disruption technique. Nakano et al17 reported a 91.2% recanalization rate by direct PTA for MCA trunk occlusion. In their report, the clinical outcome was significantly better, and massive hemorrhage was significantly lower than that in the LIT series.
However there are some potential risks associated with direct PTA, including arterial rupture, injury of the perforating artery, and spasm. Our new procedure to disrupt the MCA thrombus, the use of a deflated microballoon instead of a microcatheter, can prevent the potential risks of direct PTA and accomplish clinical results comparable to a direct PTA. Mechanical penetration by using a microcatheter is not efficient to disrupt the embolus because the area of the tip of the catheter is small.26 A microballoon has more surface area compared with a microcatheter. A deflation of the microballoon after the inflation creates more surface area and an irregular surface shape, which makes it possible to disrupt the embolus effectively. Similar to the direct PTA, the total urokinase dose is needed for a successful recanalization, thereby reducing the risk of intracerebral hemorrhage (ICH). Indeed, none of the patients in the present study experienced ICH, and of the 4 patients with M1 proximal occlusion, only 1 demonstrated infarction in the basal ganglia. This might be explained by the balloon effectively removing the embolus beyond the orifice of the LSAs and little urokinase infused into the LSAs, and unlike the direct PTA, a dilation force causing injury to LSAs was not added.
Our study has some limitations. First, it was a preliminary study and our sample size was small. Second, it was a retrospective study. Third, the neurologic examinations were performed by a neurosurgeon substituting for a neurologist. Yet our clinical results were compared with published data for LIT, other variable mechanical disruption techniques, and intravenous rtPA infusion. The comparison showed that our method may be an alternative option to LIT, previous mechanical disruption including PTA, and intravenous rtPA.
References
- Received February 23, 2006.
- Accepted after revision May 4, 2006.
- Copyright © American Society of Neuroradiology