Abstract
SUMMARY: Although the concentration of N-acetylaspartate (NAA) is often used as a neuronal integrity marker, its normal temporal variations are not well documented. To assess them over the 1–2 year periods of typical clinical trials, the whole-brain NAA concentration was measured longitudinally, over 4 years, in a cohort of healthy young adults. No significant change (adjusted for both sex and age) was measured either interpersonally or intrapersonally over the entire duration of the study.
Proton MR spectroscopy (1H-MR spectroscopy) is often used to increase the specificity of MR imaging with the levels of several detectable brain metabolites.1 Key among them is N-acetylaspartate (NAA), which is second only to glutamate as the most abundant amino acid in the mammalian brain.2 Because it is almost exclusive to neuronal cells,3,4 it is regarded as a marker for their health and concentration,1,5 and 1H-MR spectroscopy, therefore, is often part of clinical studies of neurologic disorders.6–8 Cost and complexity, however, often restrict such studies to 1–2 years, with many months between samples.9
Because when attempting to quantify neurodegeneration the NAA level of controls is frequently the implicit reference, it is important to establish its normal temporal variations in healthy subjects, that is, its sensitivity. Unfortunately, most 1H-MR spectroscopy studies to date used either single-voxel or 2D multivoxel volumes of interest.10 Covering only 10% or less of the brain, they required image-guidance to visible pathology and, therefore, are susceptible to misregistration errors in serial studies; implicitly assume changes to occur only at MR imaging-visible abnormalities; and preclude studies near the skull to prevent lipid contamination, excluding most of the cortex.11 Consequently, despite many reports on NAA variations in different diseases and brain regions, to our knowledge, its normal global temporal variations are unknown.
To test the hypothesis that the NAA level of healthy adults is stable, the present study addressed all 3 of the above issues by following whole-brain (WBNAA) concentration serially, at 2–3 time points, over 2–4 years. Such durations frequently coincide with patients’ follow-up schedule in many chronic neurologic disorders and are common in their treatment clinical trials.
Experimental Design
Human Subjects
Fourteen healthy volunteers (9 women) 29.6 ± 8.6 years old (range, 18–50 years), were recruited. “Healthy” was deemed based on negative answers to a questionnaire listing 28 neurologic disorders and unremarkable T1- and T2-weighted MR imaging, as determined by a neuroradiologist (R.I.G.). MR imaging and WBNAA were performed on each, at enrollment, 20 ± 6 months later, and for 7 of the 14 an additional 21 ± 3 months after the second time point. All of the subjects gave institutional review board-approved written informed consent.
MR Imaging and Brain Volume
All of the experiments were done on a Vision 1.5T whole-body clinical MR imager (Siemens, Erlangen, Germany) using its standard, manufacturer provided, circularly polarized volume head coil. Each subject's brain volume (VB) was obtained from the sagittal T1-weighted magnetization-prepared rapid acquisition of gradient echo (TE/TR/TI: 7.0/14.7/300 ms; 128 sections; 1.5-mm thick each; 256 × 256 matrix; 210 × 210 mm2 FOV) imaging using our MIDAS package, as described elsewhere.12
MR Spectroscopy: WBNAA Quantification
The total amount of brain NAA (QNAA) was measured in a 1.5T Vision imager (Siemens). Shimming to a consistent 15 ± 4 Hz whole-head water line was followed by nonlocalizing TE/TI/TR at 0/0.97/10 seconds for 1H-MR spectroscopy.13 Absolute quantification was done by phantom replacement against a reference 3-L sphere of 1.5 × 10−2 moles of NAA in water. Subject and reference NAA peaks, SS and SR, were integrated and QNAA obtained as follows13: 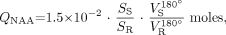 1) where VR180° and VS180° are the transmitter voltages into 50 Ω for nonselective 1-ms 180° inversion pulses on the reference and subject, reflecting relative coil loading.
1) where VR180° and VS180° are the transmitter voltages into 50 Ω for nonselective 1-ms 180° inversion pulses on the reference and subject, reflecting relative coil loading.
To account for natural brain size variations, the global NAA concentration, a specific volume-independent metric suitable for cross-sectional comparison, was used: 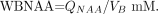 2) Intrasubject and intersubject variability have previously been shown to be better than ±7%.11,13
2) Intrasubject and intersubject variability have previously been shown to be better than ±7%.11,13
Statistical Analyses
The intrasubject change in WBNAA between each pair of time points was computed as the value at the second time point minus the first, expressed as a percentage of the value at the first. Two time points were compared with respect to the mean level and between-subject WBNAA variance. Restricted maximum likelihood was used to estimate within- and between-subject variance components within a mixed model analysis of variance framework that modeled WBNAA as a function of subject identification and time point, represented as random and fixed classification factors, respectively. A paired sample t test was used to compare time point pairs with respect to the mean WBNAA level.
The estimated between- and within-subject variance components and the observed correlation between longitudinal measures on a subject were used to compute the precision that can be expected when the yearly rate of WBNAA change is estimated using a linear mixed-model regression analysis with data from K equally spaced time points over 2 years for each of N subjects. This permitted us to determine the N needed to detect any specific annual rate of WBNAA change with either 80% or 90% statistical power at the 2-sided 5% significance level.
Results
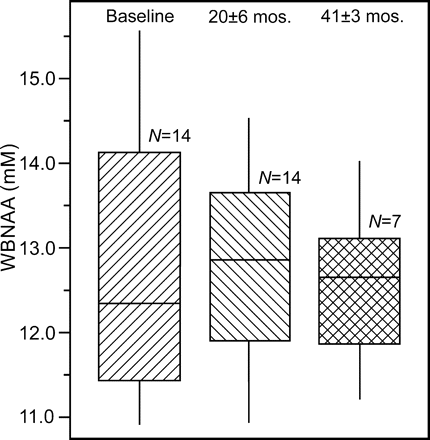
Intrasubject VB changed by an annual average of −0.25%, consistent with previous reports.14 Box plots of the WBNAA concentrations, shown in Fig 1, exhibit very similar median NAA levels between the time points with average ± SD of 12.5 ± 1.4, 12.8 ± 1.4, and 12.6 ± 1.2 mmol/L, at baseline, first, and second follow-ups, respectively.
Box plots showing the first, second (median), and third quartiles (box), ±95% (whiskers), of the WBNAA distributions at baseline, first, and second follow-up time points (the 7 subjects at the third time point are a subset of the original 14).
The intersubject distribution of WBNAA values at baseline was not different from the first or second follow-ups with respect to its mean (P > .5) or variation (P > .3). The mean intrasubject WBNAA level changes, 1.7% ± 9.0% and 0.5% ± 12% from baseline to first and second follow-up (n = 14 and 7, respectively) and −0.3% ± 9.0% between second and third time points (N = 7) were similar to previous reports for back-to-back and day-to-day changes.13 Based on this variability, estimates of the number of subjects needed to detect various annual percentages of WBNAA changes sampling K equally spaced time points over 2 years are compiled in the Table.
Estimated sample sizes for 80% or 90% power at 2-sided 5% significance level to detect specific yearly rates of WBNAA change with K (≥3) equally spaced scans per subject over 2 years
Discussion
This study quantifies, to the best of our knowledge, for the first time, the longitudinal course of the global brain NAA concentration in the healthy human brain over a 1- to 2-year period typical of most clinical trials of neurologic disorders. The results support the hypothesis that the concentration and health of neuronal cells are stable in this 20- to 50-year-old population to within the ±6%–8% precision of the WBNAA method,13 regardless of exact age or sex. Because WBNAA is a ratio (see Eq 2) and its denominator, VB, is stable to within ±0.25%,14 the intersubject and intrasubject variability can be assigned entirely to the QNAA quantification.
Knowledge of these variations can be used to aid the design of trials in which NAA is used as a surrogate marker. Specifically, how many measurements, on how many subjects, for how long, are needed to detect specific change? The Table shows, for example, that 3 measurements over 2 years on 11 patients suffices to establish 6% annual change with 90% power.
The WBNAA approach also has 3 main unavoidable limitations. First, it is inherently nonlocation specific. Second, the age range, 18–50 years, studied here is most appropriate for neurologic disorders that afflict younger patients. Finally, because NAA decline has been associated with both neuronal loss and/or dysfunction, though its changes are specific to this cell type, they are not deterministic of the type of damage.
Footnotes
This research was supported by National Institutes of Health grants EB01015, NS050520, CA92547, and NS29029.
References
- Received March 27, 2007.
- Accepted after revision May 1, 2007.
- Copyright © American Society of Neuroradiology