Abstract
BACKGROUND AND PURPOSE: Conventional angiography has been historically considered the gold standard for the diagnosis of cervical artery dissection, but MR imaging/MR angiography (MRA) and CT/CT angiography (CTA) are commonly used noninvasive alternatives. The goal of this study was to compare the ability of multidetector CT/CTA and MR imaging/MRA to detect common imaging findings of dissection.
MATERIALS AND METHODS: Patients in the data base of our Stroke Center between 2003 and 2007 with dissections who had CT/CTA and MR imaging/MRA on initial work-up were reviewed retrospectively. Two neuroradiologists evaluated the images for associated findings of dissection, including acute ischemic stroke, luminal narrowing, vessel irregularity, wall thickening/hematoma, pseudoaneurysm, and intimal flap. The readers also subjectively rated each vessel on the basis of whether the imaging findings were more clearly displayed with CT/CTA or MR imaging/MRA or were equally apparent.
RESULTS: Eighteen patients with 25 dissected vessels (15 internal carotid arteries [ICA] and 10 vertebral arteries [VA]) met the inclusion criteria. CT/CTA identified more intimal flaps, pseudoaneurysms, and high-grade stenoses than MR imaging/MRA. CT/CTA was preferred for diagnosis in 13 vessels (5 ICA, 8 VA), whereas MR imaging/MRA was preferred in 1 vessel (ICA). The 2 techniques were deemed equal in the remaining 11 vessels (9 ICA, 2 VA). A significant preference for CT/CTA was noted for VA dissections (P < .05), but not for ICA dissections.
CONCLUSION: Multidetector CT/CTA visualized more features of cervical artery dissection than MR imaging/MRA. CT/CTA was subjectively favored for vertebral dissection, whereas there was no technique preference for ICA dissection. In many cases, MR imaging/MRA provided complementary or confirmatory information, particularly given its better depiction of ischemic complications.
Dissection of the extracranial arteries accounts for 10%–25% of strokes in young and middle-aged patients.1 It may be spontaneous or traumatic and can cause a variety of clinical presentations, including stroke, headache, neck pain, tinnitus, Horner syndrome, and cranial neuropathies.1-4 Dissections are typically diagnosed on the basis of a combination of the clinical presentation, imaging studies (conventional angiography, CT/CT angiography [CTA], MR imaging/MR angiography [MRA], and sonography), and exclusion of other arterial disease, particularly atherosclerosis.1,2,5 In patients with mild or atypical symptoms, noninvasive imaging may facilitate earlier diagnosis and prevent embolic ischemic complications with the use of antithrombotic therapy.3,6
Conventional angiography has historically been considered the gold standard for dissection diagnosis, but it has limitations, which include its cost and invasiveness.1,7,8 Although the angiographic appearance of dissection is often characteristic, it does not assess the vessel wall for intramural hematoma; because of this feature, dissections in unusual locations or with atypical morphology may be misclassified or attributed to other processes. MR imaging/MRA and CT/CTA have emerged as viable alternatives for both diagnosis and follow-up of dissection.9-15 In general, the 2 techniques have different strengths and weaknesses. Diffusion-weighted MR imaging is a powerful technique for detecting acute stroke,16 which may then lead to increased scrutiny of the upstream arterial tree. Axial T1-weighted fat-suppressed images can detect the methemoglobin of the intramural hematoma within the false lumen, a finding referred to as a crescent sign.9,13 Multidetector CT/CTA has enabled routine acquisition of thinner sections with rapid imaging times, facilitating multiplanar and volume reconstructions. Additionally, it is more widely available (especially at night), has fewer contraindications, and provides greater spatial resolution than MR imaging/MRA. However, the use of ionizing radiation in the relatively young population of patients with dissection is potentially concerning, especially given the often frequent follow-up studies in these patients.
Only 1 study to date has compared CT/CTA with MR imaging/MRA for evaluation of dissection, retrospectively reviewing 7 internal carotid dissections and showing CTA to be marginally more effective in the identification of the dissected vessel.11 To our knowledge, this current study is the largest comparative series of cervical artery dissection imaged with both tomographic techniques. Given the higher spatial resolution of CT/CTA, we hypothesized that CT/CTA may be particularly suited to visualize dissections within the smaller vertebral arteries. Additionally, we hypothesized that in a series of known dissections, CT/CTA would be more sensitive to identify specific imaging features associated with dissections.
Materials and Methods
Patient Population
The study was approved by our institutional review board. The data bases of our Stroke Center, which include patients seen in both inpatient and outpatient settings, were searched from January 2003 to March 2007 for patients identified as having a dissection. Patients with definitive clinical and supportive radiologic evidence of an extracranial internal carotid artery (ICA) or vertebral artery (VA) dissection and a final diagnosis of dissection were included for analysis. Patients whose diagnosis was “possible” dissection or those in whom atherosclerotic disease was also considered a potential etiology were excluded. Only patients with both CT/CTA and MR imaging/MRA examinations on initial work-up for clinical purposes were included. Clinical records in both hard copy and electronic forms were used to extract information.
CT/CTA Parameters
Multidetector CT/CTA was performed on a 16-detector scanner (LightSpeed; GE Healthcare, Milwaukee, Wis). Noncontrast head images were obtained from the vertex to the skull base (5-mm section thickness, 120 kV, 400 mA). This was followed by CTA (0.625-mm section thickness, 140 kV, 250 mA, 1-second per rotation, 10–20 seconds to acquire images from the aortic arch to the vertex). The scanning was triggered by the CT technologist on the basis of contrast enhancement in the aortic arch following administration of 120 mL of iohexol with a concentration of 350 mg I/mL (Omnipaque 350; GE Healthcare, Princeton, NJ) at a rate of 4–5 mL/s. The CT source images were postprocessed to create contiguous coronal and sagittal reformatted images with a 2-mm section thickness, maximum intensity projection (MIP) images, volume-rendered 3D images, and curved planar reformatted images of the bilateral common and internal carotid arteries and VAs. The typical dose-length product for the noncontrast head and the contrast head and neck CTA was 1100 mGy-cm and 2200 mGy-cm, respectively. With conversion factors from the European Commission,17 this corresponds to an effective dose equivalent of 2.5 and 11.9 mSv, respectively, leading to a total effective dose equivalent for the CT/CTA protocol of 14.4 mSv.
MR Imaging/MRA Parameters
MR imaging/MRA was performed at 1.5T (Signa; GE Healthcare) by using a protocol that included diffusion-weighted imaging (DWI) of the brain with an isotropic b value of 1000 s/mm2, axial T1-weighted fat-suppressed images of the neck (TR/TE, 600/14; FOV, 20 cm; 256 × 192 matrix; 5-mm skip 1 mm; chemical-shift frequency-based fat saturation), 2D time-of-flight MRA of the neck (TR/TE, 26/4.9; FOV, 18 cm; 256 × 128 matrix; section thickness, 1.5 mm, acquired superior to inferior; superior saturation band applied), and coronal contrast-enhanced MRA of the neck using 0.1 mmol/kg of gadolinium-based contrast agent (either gadodiamide [Omniscan; GE Healthcare] or gadopentetate dimeglumine [Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, NJ]) (TR/TE, 6.5/1.4; FOV, 30 cm; 448 × 192 matrix; section thickness, 1.8 mm; flip angle, 45°; acquisition triggered by the technologist based on contrast enhancement in the aortic arch). MR angiographic sequences were postprocessed to create cutout MIP images with axial rotation. It is the standard practice of our institution to obtain axial T1 fat-suppressed images of the neck only in patients clinically suspected of having an extracranial dissection.
Image Evaluation
Imaging studies were reviewed on a PACS workstation independently by 2 neuroradiologists who were provided the patient's clinical history; these studies were typically evaluated in the order of acquisition and at the same sitting. Imaging studies were evaluated for the presence of acute ischemic stroke, vessel stenosis or occlusion, and vessel wall abnormalities, including irregularity, intimal flap, pseudoaneurysm, and intramural hematoma. This latter finding was evaluated as enlargement of the outer diameter of the vessel on CTA and as a methemoglobin crescent on axial T1-weighted fat-suppressed images for MR imaging. The 2 imaging techniques were then compared subjectively to determine which one was preferred for making the diagnosis of dissection. The final determination of technique preference took into account all of the images available with each technique (eg, CT/CTA would include noncontrast CT, CTA source images, sagittal and coronal reformatted images, curved planar reformatted images, and volume-rendered images; MR imaging/MRA would include all angiographic and anatomic imaging sequences [including DWI] and axial T1-weighted fat-suppressed images of the neck). Because some patients had multivessel dissection, this was performed on a vessel-by-vessel basis rather than on a case-by-case basis.
Statistical Analysis
Results for each neuroradiologist were compared, and κ statistics were used to measure interobserver agreement. Because interobserver agreement proved to be high, the few discrepancies were then resolved by mutual consensus. The Fisher exact test was used to determine statistical significant for technique preference. The remainder of the results are descriptive.
Results
Twenty-one patients with dissection who underwent both MR imaging/MRA and CT/CTA were identified. Three of these patients were excluded from the study due to the lack of axial T1-weighted fat-suppressed images, which were deemed of critical importance for the MR imaging/MRA evaluation of dissection. No patients were excluded due to an inadequate CT/CTA. Thus, 18 patients (10 men, 8 women; 42 ± 11 years of age) with 25 dissected vessels (15 ICAs and 10 VAs) were included in the study (Table 1). The patients were equally split between traumatic and spontaneous dissections. Four patients had multivessel dissections. CT was performed before MR imaging in 11 patients, on the same day as MR imaging in 4 patients, and after MR imaging in 3 patients. No studies were >4 days apart. Only 1 patient underwent conventional angiography, primarily to evaluate a possible cavernous-carotid fistula in the setting of a traumatic dissection rather than to characterize the ICA dissection.
Patient demographics, imaging findings, and technique preferences for dissections imaged with both CT/CTA and MR imaging/MRA
Overall, agreement between the 2 neuroradiologists regarding the presence of imaging findings of dissection (vessel patency, wall irregularity, intimal flap, pseudoaneurysm, and intramural hematoma) was high (225/250, κ = 0.80). Individual κ values ranged between 0.25 and 0.90 (Table 2), with the exception of CT/CTA vessel wall irregularity (κ = −0.04). In this case, there was high agreement (23/25, 92%), but κ was low because the finding was seen in virtually every patient; thus the agreement did not rise above that predicted by chance. Table 3 shows the frequency of imaging findings compatible with dissection observed with each technique. Overall, 78% of patients (14/18) presented with strokes that were visible on MR diffusion-weighted images. Of these, 3 were not apparent on noncontrast CT and another was very subtle.
Agreement between reviewers*
Summary of specific imaging findings of dissection by technique
Three vessels were occluded on both CTA and MR imaging/MRA (2 VA and 1 ICA). Three additional vessels that were considered occluded on MR imaging/MRA demonstrated long-segment high-grade stenosis (string sign) on CTA (2 VAs and 1 ICA). CTA did not demonstrate vessel occlusion without corresponding occlusion based on MR imaging/MRA. All dissected vessels except 1 showed irregularity on CTA. In 6 of these cases (25%), MR imaging/MRA did not show definite vessel irregularity. Pseudoaneurysms were identified on both CTA and MR imaging/MRA in 2 patients (both ICA) and on CTA alone in an additional 4 patients (1 VA, 3 ICAs). Intimal flaps were seen on CTA in 8 vessels (1 VA, 7 ICAs), none of which were identified on MR imaging/MRA. Vessel wall thickening was shown with CTA in 23/25 dissected vessels. Most of these vessels demonstrated a “methemoglobin crescent” sign on MR imaging; however, in 5 of these vessels (22%), a crescent sign was absent (4 VAs, 1 ICA). One case without obvious wall thickening on CTA did show a crescent sign. Only 1 dissected vessel showed neither wall thickening on CTA nor a methemoglobin crescent sign.
Agreement between reviewers for the preferred technique (CT/CTA, MR imaging/MRA, or equal) was 18/25 (72%). In 1 case, 1 reviewer thought CT/CTA was superior and the other preferred MR imaging/MRA; the consensus in this case was for CT/CTA. All of the remaining disagreements (6 cases) consisted of 1 reviewer considering CT/CTA superior and the other considering the 2 techniques equivalent. In 4 of these cases, consensus was that the 2 techniques were equal, whereas in the remaining 2 cases, the consensus was that CT/CTA was preferred.
CT/CTA was considered the preferred technique in 5 of the 15 ICA dissections and in 8 of the 10 VA dissections (Table 4). A typical case in which CT/CTA was the preferred technique is shown as Fig 1. MR imaging/MRA was preferred in only 1 case, an ICA dissection that demonstrated a clear crescent sign (Fig 2). The 2 techniques were deemed equivalent for diagnosis in the remaining 11 vessels (Fig 3). The preference for CT/CTA was significant for VA dissections (P = .041, Fisher exact test), but there was no preference for ICA dissections. Figures 4 and 5 depict several relevant clinical situations: a long-segment high-grade stenosis (string sign) seen on CTA, which appeared occluded on MR imaging/MRA, and a pseudoaneurysm seen only on CTA, respectively.
CT/CTA imaging of multivessel dissection (right ICA and VA) provides more detailed information than MR imaging/MRA about vessel morphology. Conventional cerebral angiographic images of the right ICA (A) and right VA (B). Curved planar reformat images from the CTA study of the right ICA (C) and right VA (D). Both demonstrate vessel wall irregularity, mild luminal narrowing, and pseudoaneurysm of the right ICA (arrows). E, Corresponding MIP image from contrast-enhanced MRA shows both dissections (arrows), but with less clear depiction than that on CTA. F, Axial T1-weighted fat-suppressed image demonstrates a crescent sign consistent with intramural hematoma around both the right ICA and VA (arrows).
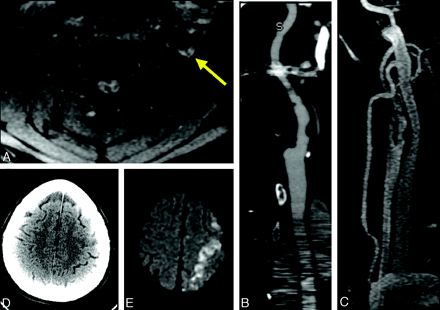
CT/CTA and MR imaging/MRA of a left ICA dissection provide similar information about vessel morphology, but MR imaging is deemed superior for making the diagnosis, particularly because of detection of acute stroke. A, Axial T1-weighted fat-suppressed image demonstrates a crescent sign around the left ICA (arrow). B, CTA curved planar reformat shows irregularity and thickening of the left ICA wall with calcified plaque. These findings are not specific for dissection and could be due to atherosclerosis. C, Contrast-enhanced MRA MIP reformat demonstrates the similar appearance of the irregular left ICA. D and E, Noncontrast CT (D) does not show the acute infarct, which is clearly identified in the left middle cerebral artery–anterior cerebral artery watershed territory on DWI (E).
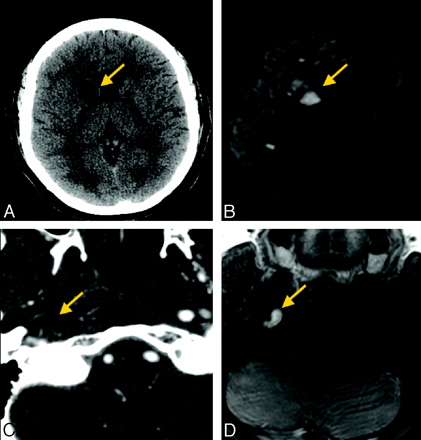
CT/CTA and MR imaging/MRA provide different advantages in this case of right ICA dissection, but diagnosis is made with equal confidence on both techniques. A and B, Noncontrast CT image (A) shows mild hypoattenuation involving the right basal ganglia (arrow), confirmed as an acute stroke with DWI (B). C, Axial source image from CTA shows expansion of the outer wall of the right ICA consistent with wall thickening as well as narrowing of the vessel lumen (arrow). D, Axial T1-weighted fat-suppressed MR image demonstrates a crescent sign (arrow) around the right ICA.
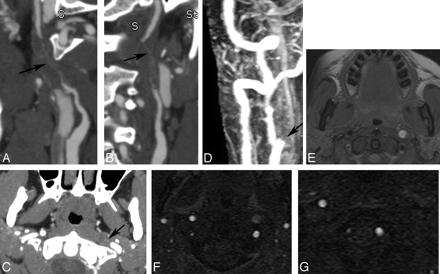
Example of a long-segment high-grade stenosis (string sign) identified on CTA, which appeared occluded on MR imaging/MRA. A and B, Curved planar reformatted images of the left ICA demonstrate flamelike tapering of the proximal vessel with wall thickening and long-segment high-grade stenosis (arrow). C, Axial plane from CTA shows the tiny residual lumen of the vessel (arrow). D, Contrast-enhanced MRA acquired on the same day demonstrates apparent occlusion just distal to the carotid bifurcation (arrow). E, T1-weighted fat-suppressed axial image demonstrates methemoglobin in the left ICA wall. F and G, Axial source images from 2D time-of-flight MR angiography show the T1 shinethrough of the methemoglobin, but the absence of flow-related enhancement in the left distal cervical ICA.
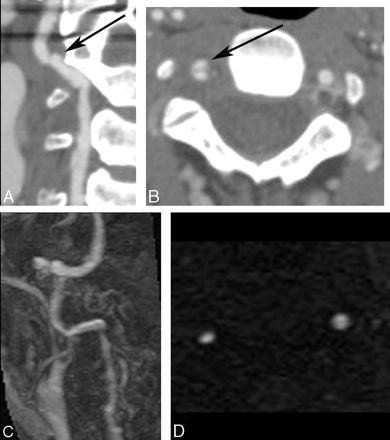
Right vertebral artery dissection with a pseudoaneurysm seen on CTA, which is not visualized on MR imaging/MRA. A, Curved planar reformatted image from CTA of the distal right vertebral artery demonstrates irregularity (arrow) as well as a small distal pseudoaneurysm. B, An axial CTA source image shows contrast within the true lumen and the pseudoaneurysm anteriorly (arrow). C, Contrast-enhanced MRA demonstrates the irregularity associated with the dissection, but not the pseudoaneurysm. D, 2D time-of-flight MRA depicts the narrowed true lumen but does not show the pseudoaneurysm, presumably due to saturation of slow-flowing blood.
Technique preference by vessel location*
Discussion
Conventional angiography has long been considered the gold standard for evaluation of extracranial arterial dissection, but MR imaging/MRA has gradually replaced conventional angiography in this role.1 However, MR imaging is known to be less sensitive for vertebral artery dissection than for carotid dissection,9 probably due to the small size of the vertebral arteries and difficulties with distinguishing a methemoglobin crescent sign from normal epidural venous plexus. Additionally, access to MR imaging can be problematic, either due to cost, availability, or patient restrictions (eg, pacemakers, claustrophobia, etc). Also, the proper MR imaging protocol (including axial T1-weighted fat-suppressed images) may not be performed if dissection is not suggested clinically. In our series, we found that 3 of 21 patients had to be excluded because T1-weighted fat-suppressed images of the neck were not acquired. There is limited literature regarding the efficacy of CT/CTA for the diagnosis of dissection, probably because of the relatively recent introduction of rapid multidetector CT systems capable of thin sections.10-12,18 This study sought to examine the relative strengths and weaknesses of the 2 techniques in patients with dissection undergoing both studies in the acute time period and to determine whether one or the other technique was preferred, depending on whether the dissected vessel was in the anterior or posterior circulation.
This retrospective study cannot, by its nature, comment on which technique would be more sensitive for dissection findings in an unknown case; however, because not all the signs of dissection need be present to make the diagnosis, we thought it would be informative to determine which technique identified more dissection features in a group of patients with known dissection, with the idea that the use of such a technique could increase confidence in this often challenging diagnosis. First, we found a high level of agreement between 2 neuroradiologists regarding the presence of specific signs of dissection. Many of these signs were more frequently identified with CT/CTA, perhaps due its higher spatial resolution (Table 2). This was true in both the anterior and posterior circulations. In particular, we found that intimal flaps and pseudoaneurysms were much less frequently seen with MR imaging/MRA. As expected, DWI allowed better visualization of acute ischemic stroke. We believe that this finding is often of critical importance because it may prompt more careful attention to the upstream arteries and dedicated imaging of the neck vessels in patients in whom dissection was not initially considered on clinical grounds.
We found that when there was a preference for technique, it was almost always in favor of CT/CTA. This preference is likely due to the ability of CT/CTA to identify more imaging features associated with dissection. For example, intimal flaps were observed in 7 vessels with CT/CTA, none of which were visible on MR imaging. Also, of the 6 pseudoaneurysms identified with CT/CTA, only 2 (33%) were identified with MR imaging/MRA. Finally, we found that CT/CTA detected a tiny residual patent vessel lumen (string sign) in 3 of 6 cases (50%) that were read as occluded on MR imaging/MRA. Clinical management decisions, including possible interventional procedures, can depend on the presence or absence of pseudoaneurysms or very high-grade stenoses (versus occlusions), which may represent potential sources for emboli; thus, the higher sensitivity of CTA for these findings may be required by neurologists, even for dissections that are diagnosed initially with MR imaging/MRA.
Only in 1 of 18 cases (6%) was MR imaging/MRA considered the preferred technique. This case of ICA dissection (Fig 2) demonstrated a classic crescent sign and ipsilateral acute ischemic stroke. In half of the remaining cases, CT/CTA was preferred over MR imaging/MRA. There was a significant preference for CT/CTA in the setting of vertebral dissection (P = .041, Fisher exact test). Specifically, we found twofold increased preference for CT/CTA for cases of VA dissection. This finding is consistent with the initial report of Kurokawa et al12 and the more extensive study performed by Chen et al,10 who demonstrated high sensitivity and specificity for 17 patients with vertebral dissections, by using conventional angiography as a gold standard. For ICA dissections, the 2 techniques were deemed equivalent 80% of the time. Early studies of helical CTA in the evaluation of ICA dissections were encouraging but limited by the CT technology at the time and required a priori knowledge of the location of the vessel wall abnormality.19,20 More recently, Elijovich et al11 reported that 5 of 7 cases of ICA dissection were identified with both CT/CTA and MR imaging/MRA, implying that though there may be a slight preference for CT/CTA in the anterior circulation, in most cases, both techniques arrived at the same diagnosis.
Our study highlights the usefulness of demonstrating intramural hematoma, either as wall thickening on CTA or as a methemoglobin crescent on axial T1-weighted fat-suppressed images, to diagnose dissection.9 The evaluation of intramural hematoma with each technique has its own drawbacks. Wall thickening was detected on most of the CT/CTAs in our study but is considered a less specific finding than a methemoglobin crescent and is not always as conspicuous to the radiologist reviewing the images. A crescent sign was seen in 19/25 dissections (76%), which may be related to the timing of patient presentation. Evolution of the hematoma within a vessel wall has not been extensively studied, but it is possible that high T1 signal intensity may not be present during the early acute period.15,21,22 T2-weighted sequences (with or without fat saturation) may add sensitivity for the diagnosis of dissection in the setting of absent or equivocal findings on T1-weighted imaging, to evaluate hyperacute blood or wall edema.
The axial T1 fat-suppressed sequence can be further confounded by sources of bright signal intensity other than intramural hematoma, including epidural venous plexus around the vessel, slow flow, tortuosity, failure of adequate fat suppression, and/or postcontrast imaging due to technologist error.14
Our study has several limitations. There is an inherent bias in retrospective reviews of case series. Cases in which both CT/CTA and MR imaging/MRA were performed may have been more complex than those evaluated with either technique alone. Further, the separation of studies in time may have allowed certain findings (such as those of acute cerebral infarction) to evolve and become more or less apparent. In addition, the neuroradiologists reviewing the studies were not blinded to the clinical history, and the images were reviewed primarily in the order they were acquired (leading to potential bias). In most of the patients, CT/CTA was performed first, and thus a preference for CT/CTA based on prior information from the MR imaging/MRA study would be unlikely. Likewise, because there were more imaging findings on CT/CTA than on the MR imaging/MRA studies reviewed afterward, we do not believe that such bias played a large role in this study.
Control cases were not included. Although this limited our ability to comment on the diagnostic capability of each technique, the goal was to determine whether specific findings that are commonly found in dissections were present and to determine a preferred technique based on the perceived ease of identifying and characterizing the dissected vessels. As mentioned previously, given that only 1 patient received a catheter angiogram, definition of an independent gold standard for these imaging findings was not possible. A study with angiographic correlates in large numbers of patients would be difficult to undertake in the current practice environment, given the increasing reliance on noninvasive imaging techniques for diagnosing dissection. Finally, there was no attempt made to optimize either the CT/CTA or MR imaging/MRA sequences to improve sensitivity to signs of dissection; instead, we focused on how common imaging protocols fared in routine clinical practice. Last, such a comparative study (without a gold standard reference) is prone to errors of specificity in that one can never definitively distinguish an artifact from a true finding.
Our study highlights the often complementary advantages of MR imaging/MRA and CT/CTA in the frequently challenging diagnosis and assessment of cervical artery dissection. Although 1 recent review article4 suggested exclusive use of MR imaging/MRA in the initial diagnosis of dissection, with conventional angiography or CT/CTA reserved for nondiagnostic MR imaging studies, our study suggests that MR imaging/MRA alone may be less sensitive for important features of dissection, such as pseudoaneurysm and long-segment high-grade stenosis, which may impact potential treatment. Overall, we found that CT/CTA demonstrated most common dissection findings more frequently than MR imaging/MRA, with the important exception of acute ischemic stroke. Given the well-recognized superiority of MR imaging/MRA to depict ischemia and intramural hematoma, it might be possible to shorten the MR imaging protocol in patients with a high suggestion of dissection based on prior CT/CTA, to include only DWI and axial T1-weighted fat-suppressed images. Also, patients with clinically suggested ICA dissection (eg, anterior neck pain, Horner syndrome, monocular vision abnormalities, etc) may be initially evaluated with MR imaging/MRA, given the lack of ionizing radiation and similar conspicuity of common dissection imaging findings. Practical considerations, such as technique availability, specific contraindications (eg, pacemakers, contrast allergies, renal function, pregnancy, etc), and patient tolerance (eg, claustrophobia) will also likely dictate the primary noninvasive imaging technique used. Finally, although this study highlights the decrease in the use of conventional angiography for the diagnosis of dissection, it may continue to be helpful in confusing or ambiguous cases.
References
- Received February 29, 2008.
- Accepted after revision May 3, 2008.
- Copyright © American Society of Neuroradiology