Abstract
BACKGROUND AND PURPOSE: Several studies have questioned the traditional belief that the corticospinal tract (CST) arises exclusively from the precentral gyrus and passes through the anterior half of the posterior limb of the internal capsule (PLIC) in humans; however, no direct evidence existed from structural imaging, and developmental aspects of CST origin have not been clarified. We used diffusion tensor imaging (DTI) tractography to test the hypotheses that CST can originate from both pre- and postcentral gyri and is located posteriorly in the PLIC, and we also determined how age, sex, or handedness affected these locations.
MATERIALS AND METHODS: Forty-two healthy children (2.6–17.5 years of age; 20 girls) underwent DTI. Subsequently, tractography was performed on the basis of fiber assignment by continuous tracking (FACT) algorithm and brute force approach, with a fractional anisotropy (FA) threshold of <0.2 and an angle threshold of >50°. The CST was isolated by using a knowledge-based region-of-interest approach, and its cortical origin and location on the PLIC was determined.
RESULTS: DTI revealed that the CST originated from both pre- and postcentral gyri in 71.4% of hemispheres, from the precentral gyrus only in 19%, and from the postcentral gyrus only in 7.1%. The overall distribution was similar in both hemispheres. However, children with CST originating from both pre- and postcentral gyri were older (mean, 11.1 years of age) than those with precentral origin (mean, 5.8 years of age) or postcentral origin (mean, 7.8 years of age) only (P = .00003). The center of the CST was localized at 65% of the length (from its anterior margin) of the PLIC, and the CST occupied 26.5% of its anteroposterior length. There was a significant positive correlation between age and FA of the CST (r = 0.49; P = .002). The volume of the precentral portion of the left CST was significantly higher than that of its postcentral portion (P = .01) and that of the right CST (P = .0002). The pattern of cortical origin of CST, its location at the level of PLIC, and its volume and FA were unaffected by sex or handedness.
CONCLUSIONS: The CST most frequently originates from both pre- and postcentral gyri, especially in older children, and is typically centered approximately two thirds of the distance from the anterior margin of the PLIC and occupies about a quarter of its anteroposterior length. In young children, the CST can often be seen originating exclusively from the precentral gyrus by DTI.
The axons of the corticospinal tract (CST) have traditionally been assumed to arise from neurons in the precentral gyrus (Brodmann area 4). An early human study with direct cortical stimulation most frequently but not exclusively localized the primary motor cortex in the precentral gyrus.1 However, recent studies of patients with focal epilepsy have demonstrated that motor responses can be induced by electrical stimulation of both pre- and postcentral gyri.2–5 Because direct electric stimulation cannot be performed in healthy subjects, it is unclear how much one can generalize the information gathered by cortical stimulation in patients with epilepsy, tumor, or other neurologic disorders, and it can be argued that any demonstrated pattern may be affected by the underlying pathology (because of abnormal development, reorganization, or displacement). In addition, the exact path of the CST, particularly its localization in the internal capsule, also remains uncertain. The CST is generally believed to pass through the anterior half of the posterior limb of the internal capsule (PLIC), a classic view that goes back to the early studies of Charcot and Hadden,6 Dejerine,7 and Foerster.8 Despite many doubts raised about this classic concept by several neurosurgical and stereotactic studies,9–14 suggesting that the CST is located more posteriorly in the PLIC, many investigators continue to support the original concept, and in many anatomic illustrations and anatomy textbooks, the CST, particularly the head, neck, and upper limb fibers, are still shown in the anterior half of the PLIC.15,16 Furthermore, the exact location and spread of the CST within the PLIC have not been fully characterized.
Although several techniques, such as functional MR imaging (fMRI) or magnetoencephalography, can noninvasively demonstrate the primary motor cortex, only diffusion tensor imaging (DTI) tractography, which relies on the pattern of water diffusion to provide information about white matter tracts, can physically demonstrate both the origin and path of the CST noninvasively.17 Although a number of previous DTI studies have attempted to isolate and characterize the CST in humans,18–24 only 2 studies evaluated healthy subjects.20,23 Also, in these studies the precentral gyrus was presumed to be the primary motor cortex, and a region of interest to isolate the CST was placed only in the precentral gyrus. Furthermore, none of the previous DTI studies were performed in a pediatric population; therefore, potential developmental changes were not evaluated.
In the present study, we delineated the CST by using DTI tractography in a group of healthy children, without assuming that the primary motor cortex is confined to the precentral gyrus. We hypothesized that the CST originates from both pre- and postcentral gyri in most subjects and is located posteriorly in the PLIC. We also determined how age, sex, or handedness affected these locations.
Materials and Methods
Subjects
DTI was performed in 42 healthy children with normal development (mean age: 9.7 ± 4.5 years; range, 2.6–17.5; 20 females; 33 right-handed). All healthy children were screened by a pediatric neurologist and a pediatric neuropsychologist, and none of the subjects had any historic or current medical, developmental, or psychiatric diagnosis. The study was approved by the institutional review board at Wayne State University, and written informed consent was obtained from the parents or guardians of all subjects.
MR Imaging Acquisition
All participants were scanned on 3T TwinSpeed Excite Scanner (GE Healthcare, Milwaukee, Wis) by using an 8-channel head coil. The full MR imaging acquisition included the following series: 1) 3D structural scan of the brain by using a T1-weighted inversion recovery fast-spoiled gradient recall sequence designed to optimize the tissue contrast between gray and white matter. Imaging parameters were as follows: TR/TE/TI = 6.2/2.5/450 ms; FA = 12°; matrix size = 265 × 265; array spatial sensitivity encoding technique (ASSET) factor of 2; FOV = 205 × 205 mm2, zipped by 2, with a thickness of 1.6 mm, yielding a cubic voxel of 0.8 × 0.8 × 0.8 mm3; 2) T2 fluid-attenuated inversion recovery acquired in 30 coronal planes with TR/TE/TI = 11,000/160/2450 ms; FOV = 240 × 240 mm2; matrix = 288 × 192; section thickness = 4 mm and 1-mm gap; 2 repetitions; and 3) axial T2-weighted fast spin-echo (echo-train length, 32) with TR/TE = 5000/108 ms; section thickness = 4 mm; gap = 1 mm; matrix = 384 × 256; 2 repetitions. In addition, a spin-echo echo-planar imaging (EPI) diffusion-weighted sequence was acquired in the axial plane with diffusion sensitization gradients applied in 6 noncollinear (Coulomb forces scheme) directions (Di, i = [1, …, 6]) with b-value of 1000 s/mm2.
The same imaging parameters were used to acquire T2-weighted (D0) images (b ∼ 0 s/mm2) to use as reference images and to measure the signal-intensity attenuation. All image volumes were repeated 6 × to reproduce more measurements.25–27 This increases the signal intensity–to-noise ratio and reduces image artifacts. The TE was 79 ms, and the TR was approximately 10 seconds. A set of minimum 34 axial sections of 3-mm thickness without gap was acquired with a matrix size of 128 × 128 and reconstructed to 256 × 256 matrix with homodyne, covering the whole brain including the cerebellum. The FOV was 240 × 240 mm2, and the approximate scanning time for the DTI acquisition was 9 minutes. A double refocusing pulse was used to reduce eddy-current artifacts,28 and ASSET was performed to further reduce the geometric distortion from eddy current caused by EPI. Because no major artifacts were observed, even at the level of the deep brain structures, no off-line correction was performed. The children were not sedated during MR imaging; however, younger children were scanned while sleeping and all children were monitored for movement during scanning. If there was significant movement, MR imaging was repeated or those scans were removed from the study.
Data Processing
Acquired diffusion-sensitized and reference image sets were transferred to an Intel Pentium Windows−based operating system (Microsoft, Bothell, Wash) for further data analysis. Tensor calculation and tractography were performed by using DTIStudio Software Version 2.40 (www.mristudio.org). All diffusion-weighted and non–diffusion-weighted images were first realigned by affine transformation by using the automated image registration program,29 to further minimize eddy current and potential small bulk motions that may have occurred during the scans. The 6 elements of the diffusion tensor were calculated for each pixel by using multivariate linear fitting.30 After diagonalization, 3 eigenvalues and 3 eigenvectors were obtained. The eigenvector associated with the largest eigenvalue was used as an indicator of fiber orientation.
Isolation of the CST on DTI Tractography
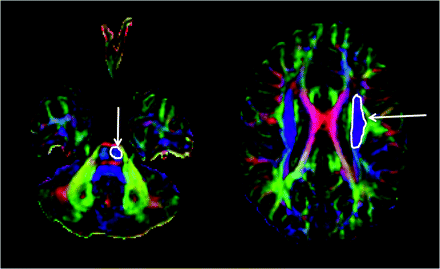
Tractography was performed on the basis of fiber assignment by continuous tracking (FACT).17 A brute force fiber tracking was initially performed for the whole brain. The fiber propagation was stopped at a fractional anisotropy (FA) threshold of <0.2 or an angle threshold >50°. The DTIStudio software allows isolation of tracts passing through a single region of interest (by using the inclusive “OR” operator) or multiple regions of interest (by using the exclusive “AND” operator). The CST was isolated by drawing an “OR” region of interest around the CST in the brain stem (identified as a blue color bundle [ie, a bundle running in the superior-inferior direction] in the anterior part of the brain stem) and an “AND” region of interest around the corona radiata in the direction-coded color axial sections (Fig 1). Unrelated fibers, such as fibers going to the contralateral side, cerebellum, or thalamus, were removed by using a “NOT” region of interest. To be included into the study and for further analysis, the CST had to be continuous from the brain stem to the motor cortex. If it was not continuous, it was considered as unidentifiable, and it was concluded that DTI failed to isolate that particular CST. The above procedure was performed independently by 2 investigators (A.K. and S.K.S.) experienced in performing tractography with DTIStudio software.
Isolation of corticospinal tract (CST) on diffusion tensor imaging tractography. The CST was isolated by drawing an “OR” region of interest (arrow) around the CST in the brain stem (identified as a blue color bundle [ie, the bundle running in the superior-inferior direction] in the anterior part of the brain stem) and an “AND” region of interest (arrow) around the corona radiata in the direction-coded color axial sections.
Identification of the Central Sulcus, Precentral Gyrus, and Postcentral Gyrus
The central sulcus was determined by the consensus of 2 investigators (E.A. and C.J.), according to the anatomic MR imaging landmarks, which have been previously validated.2,31–34 The primary criterion for defining the central sulcus included the omega-shaped sulcus at 4–5 cm above the Sylvian fissure on the horizontal plane. If there were potentially 2 sulci showing the omega shape, the sulcus just posterior to the precentral sulcus, which has a junction with the superior frontal sulcus, was defined as the central sulcus. The sulcus meeting the above criteria was determined by using the 3D Tool software (Max-Planck-Institute, Katlenburg-Lindau, Germany),35 which displayed both orthogonal (sagittal, axial, and coronal) as well as 3D brain surface images. Once the central sulcus was determined, the gyrus just anterior to the central sulcus was determined as the precentral gyrus, and the one just posterior to the central sulcus was designated as the postcentral gyrus.
Classification of the Cortical Origin of the CST on DTI Tractography
On the basis of the consensus of 2 investigators (A.K. and S.K.S.) regarding the location of the CST at the horizontal plane showing the central sulcus, each hemisphere of each subject was classified into 1 of the following 4 groups: 1) The nonlocalization group included those subjects in whom DTI failed to isolate the CST, 2) the precentral gyrus group included those who had the CST origin confined to the precentral gyrus, 3) the postcentral gyrus group included those who had the CST origin confined to the postcentral gyrus, and 4) the pre- and postcentral gyri group included those in whom the CST originated from both pre- and postcentral gyri.
In addition, the volume (total number of voxels) and FA of CST were quantitatively measured for each component of CST (1 originating from the precentral gyrus and the other originating from the postcentral gyrus) between the cortical and brain stem levels.
Measurement of the Location of the CST at the Level of the PLIC
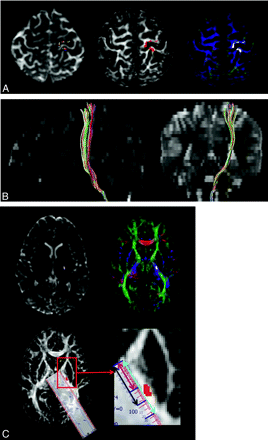
The path of the CST was determined at the horizontal plane showing the PLIC, thalamus, putamen, and globus pallidus as follows: First, the anterior margin of PLIC was defined as the medial apex of the globus pallidus, and the posterior margin of PLIC was defined as the posterior apex of the putamen. The total length of the PLIC (light blue line in Fig 2C), the distance between the anterior margin of PLIC and the anterior margin of CST (pink line in Fig 2C), and the distance between the anterior margin of the PLIC and the posterior margin of the CST (black line in Fig 2C) were measured, while keeping zooming and image parameters constant for all subjects.
Origin and course of the CST in a 12-year-old healthy girl. A, Transaxial summed B0 (T2-weighted) (left), FA (middle), and color-map (right) images show the origin of left CST from both pre- and postcentral gyri (CST shown in multicolor in the B0, in red in the FA, and in white in color-map images). B, Sagittal and coronal summed B0 images (T2-weighted) show the course of the CST from the Rolandic cortex to the brain stem. The CST originates from both pre- and postcentral gyri and involves the anterior portion of brain stem. C, Transaxial summed B0 (T2-weighted) (upper row left), color-map (upper row right), and FA (lower row left) images show location of the CST in the third quadrant of the posterior limb of internal capsule (PLIC). The internal capsule is well visualized on the FA image (white) and color-map image (PLIC is bluish because of the predominant superior-inferior direction of the CST). In the right lower row is the blown-up image of the PLIC and the schema used to measure the location and spread of CST on the PLIC. With the help of calibrated ruler software, the total length of the PLIC (light blue line), the distance between the anterior margin of the PLIC and the anterior margin of the CST (pink line), and the distance between the anterior margin of the PLIC and the posterior margin of the CST (black line) are measured. Zooming and image parameters are constant for all subjects.
From these measurements, the distance from the anterior margin of PLIC up to the midpoint of the CST (defined as the “center” of the CST in the present study) and the length of the PLIC occupied by the CST (defined as “spread” of the CST) were determined. All measurements were obtained in native space and normalized to the entire length of the PLIC in each patient to allow interindividual comparisons, irrespective of the brain size. This approach provided 2 values: 1) a percentage value for the center of the CST within the PLIC, where 0% is the anterior margin and 100% is the posterior margin of the PLIC, and 2) a percentage value for the spread of the CST, which characterized the length of PLIC occupied by the CST bundle, expressed as the percentage of the entire length of the PLIC, from the anterior margin to the posterior margin in a given axial plane.
Statistical Analysis
Values are expressed as mean ± SE; 95% confidence interval (CI) for the center and spread of the CST are also given. The χ2 test was used to evaluate the difference in the proportions of various categoric variables for ≥2 groups, and a paired t test or analysis of variance was performed to determine the difference in various continuous variables. Correlation analysis was performed to determine the correlation between age and various continuous variables. The Statistical Package for the Social Sciences, Version 16.0 (SPSS, Chicago, Ill) was used for the data analyses.
Results
Cortical Origin of the CST
Data for the cortical origin of the CST and its location in the PLIC is given in on-line Table 1. Most commonly, CST originated from both pre- and postcentral gyri (n = 60 hemispheres, 71.4%) (Fig 2A, -B), less frequently, from the precentral gyrus only (n = 16 hemispheres, 19%), and only rarely from the postcentral gyrus only (n = 6 hemisphere, 7.1%). DTI failed to isolate CST in 2 (2.4%) hemispheres, both on the left side in 6- and 11-year-old boys, in whom the right CST could be detected. The CST originated from the precentral gyrus in 7 (16.7%), the postcentral gyrus in 3 (7.1%), and both pre- and postcentral gyri in 30 (71.4%) children, in the left hemisphere; and from the precentral gyrus in 9 (21.5%), the postcentral gyrus in 3 (7.1%), and both pre- and postcentral gyri in 30 (71.4%) children in the right hemisphere. The overall distribution was not different between the left and right hemispheres (P = .52). However, cortical origin of the CST appeared to be related to age: Children with CST originating from both pre- and postcentral gyri were significantly older (mean age, 11.1 ± 4.1 years) than those with precentral origin only (mean age, 5.8 ± 4.3 years) or with postcentral origin only (7.8 ± 2.1 years) (P = .00003). This was true for CSTs in both hemispheres: The mean age of children with the CST originating from the precentral gyrus, postcentral gyrus, or both pre- and postcentral gyri was 5.4 ± 4.8, 7.2 ± 0.2, and 11.1 ± 4.0 years, respectively (P = .004), in the left hemisphere; and 6.1 ± 4.1, 8.4 ± 3.2, and 11.0 ± 4.2 years, respectively (P = .01), in the right hemisphere.
Location and Spread of the CST in the Internal Capsule
On average, the center of the CST was localized at 64% (95% CI, 62–65%) of the length of the PLIC (from its anterior margin) on the left hemisphere but slightly more posterior, at 66% (95% CI, 65–68%) of the length of the PLIC on the right (P = .04). The average spread of CST (the anteroposterior length of PLIC occupied by the CST) in the PLIC was greater in the left than in the right side, 29% (95% CI, 27–31%) versus 24% (95% CI, 21–27%), respectively (P = .001) (Fig 2C).
Volume and Diffusion Property of Different Portions of CST
Volume and FA of the pre- and postcentral portion of the CST is given in on-line Table 2. FA values showed an age-related increase; however, this correlation was significant in the precentral portion of the CST on the left side (r = 0.49, P = .002) and in the postcentral portion of the CST on the right side (r = 0.43, P = .01) only (Fig 3A). A strong age-related increase in the fiber volume was also found in the same CST portions (r = 0.48, P = .003 for the precentral portion of the left CST and r = 0.55, P = .001 for the postcentral portion of the right CST) (Fig 3B). In addition, the volume of the precentral portion of the CST was significantly larger on the left side as compared with the right in children in whom the CST originated from both pre- and postcentral gyri (P = .0002, paired t test). In contrast, the postcentral portion of the CST was similar in volume on the 2 sides (P = .82). FA values were not different between the 2 sides (On-line Table 2). Finally, among children in whom CST originated from both pre- and postcentral gyri, the volume of CST parts originating from the precentral gyrus was significantly higher than in those originating from the postcentral gyrus in the left hemisphere (P = .01). However, no similar differences were seen in the right hemisphere (P = .48). FA values were also not different between pre- versus postcentral CST portions on either side (left: P = .81, right: P = .17).
Age-related increase of FA (A) and volume of the pre- and postcentral portions of the CST in the left and right sides (B). The graph demonstrates an age-related increase of FA and fiber volume in both portions on both sides; however, statistically significant correlation is seen for the precentral portion of the left CST and the postcentral portion of the right CST only.
Effect of Sex and Handedness
There was no effect of sex or handedness on the pattern of cortical origin of the CST, its location in the PLIC, or its volume and FA value.
Discussion
The major findings of the present study are the following: 1) The cortical origin of the CST on DTI tractography frequently involved both pre- and postcentral gyri in both hemispheres; this was particularly prevalent in older children. 2) In contrast to the traditionally held notions regarding the location of the CST in the PLIC, the CST was centered around the anterior two thirds of the PLIC and occupied about one fourth of it; the left CST occupied a larger segment of the PLIC than the right CST. 3) The precentral portion of the left CST increased with age and had a larger volume than its right counterpart as well as the postcentral portion of the left CST. 4) Although the limited number of cases precludes definitive conclusions, there appears to be no effect of sex or handedness on the pattern of cortical origin of the CST, its location on the PLIC, or its volume and diffusion properties.
Localization of the Primary Motor Cortex
Despite investigations performed for more than a century, the exact localization of the primary motor cortex remains controversial. Classically, the motor cortex was thought to be located in the precentral gyrus.1 Several recent studies of patients with focal epilepsy have localized the primary motor cortex not only in the precentral gyrus but also in the postcentral gyrus.2–5 Our recent studies showed that hand motor responses could be induced by electric stimulation of the postcentral gyrus in >80% of children with focal epilepsy.2,3 A study by using the technique of retrograde labeling with horseradish peroxidase in monkeys revealed that both precentral and postcentral gyri contained corticospinal cells.36 Later, functional studies demonstrated that a lesion37 or cooling,38 confined to the presumed postcentral gyrus, resulted in hemiparesis of the contralateral upper extremity in monkeys. Neuroimaging studies by using fMRI revealed that motor tasks such as finger tapping or hand grasping consistently activated the contralateral postcentral gyrus defined by anatomic landmarks, in addition to the precentral, premotor, and supplementary motor areas.34 Therefore, our findings in a large cohort of healthy children are consistent with and confirm the observations from these studies. An advantage of our study is that in isolating the whole CST, unlike previous DTI studies regarding the CST, we did not assume a priori that the precentral gyrus is the primary motor cortex. Therefore, we believe that the present observations can be extrapolated to the healthy population with more certainty and confidence compared with previous studies.
Localization of CST in the Internal Capsule
The somatotopic organization of the CST in the PLIC has been a matter of considerable debate for more than a century. Traditionally, the CST was thought to pass through the anterior one third of the PLIC.6,7 Although many subsequent studies by using brain dissection or stereotactic procedures have challenged this view and have suggested that the CST may be localized in the posterior portion of the PLIC,9–14 the classic view is still persisting and finds mention in anatomy textbooks.16 Recent DTI studies have also challenged this old view,20,39,40 and our results, derived from a large healthy population studied without preselecting the precentral gyrus as the primary motor cortex, are consistent with a more posterior location of the CST in the PLIC. Thus, the present study has reconfirmed the findings of the previous stereotactic, neuropathologic, and macroscopic observations and provided a detailed pattern of localization and spread of the CST in the PLIC. Most interesting, the spread of the CST in the left PLIC was significantly larger than that in the right PLIC. This may be related to the higher volume of the left CST, particularly its precentral portion, when the CST originated from both pre- and postcentral gyri.
Volume and Diffusion Property
Although there was no statistical difference in FA values between the left or right CST or the pre- and postcentral portions of the CST (FA usually reflects the microstructural integrity of white matter, including myelination), the volume of the precentral portion of the left CST was larger than its right counterpart as well as the left postcentral portion. This finding is consistent with that reported by Rademacher et al,41 who showed larger volumes of the precentral portion of the CST in the left hemisphere than in the right hemisphere in a postmortem study. Similarly, by using voxel-based morphometry, Herve et al42 found higher values of white matter corresponding to the CST in the left hemisphere than in the right. Westerhausen et al43 also reported a trend toward a larger relative size of the CST in the left compared with the right hemisphere. The larger volume of the precentral portion of the CST may be related to the left hemispheric dominance, not only in right- handed but also in most left-handed subjects.
Effect of Age
Most interesting, we found that children with the CST originating from both the pre- and postcentral gyri were older than children in whom the CST originated from the precentral gyrus. Although the reason for this finding remains to be clarified, a possible explanation could be a more myelinated and mature CST, associated with more complex fine-motor activity in older children.
Many previous DTI studies have demonstrated an increase in FA and volume with age, in different parts and levels of white matter, including centrum semiovale, corona radiata, and the PLIC,43–48 which is consistent with our finding of increased volume and FA of the CST with age. However, we found this specifically in the precentral portion of the left and postcentral portion of the right CST. This difference may be methodologic, because we isolated the whole CST along with its pre- and postcentral portion, unlike in previous studies which selectively focused on white matter regions assumed to belong to the CST. However, why these changes are restricted only to specific portions of the CST is not clear to us.
Effect of Sex and Handedness
We did not find any effect of sex or handedness on the pattern of cortical origin of the CST, its location on the PLIC, or its volume and FA. Although our sample size is small to perform a conclusive subgroup analysis, particularly for handedness, our results are similar to those reported by other studies,43,46,47 which did not find any sex effect on diffusion parameters or volume for any of the white matter areas or tracts, including the CST. Significant asymmetry in the thresholds for electromyographic activation by using transcranial magnetic stimulation,49 in activation shown by fMRI50 and in motor cortex volume (gray matter),42 has been reported to be related to handedness; however, studies directly evaluating the white matter have not found the effect of handedness on the CST.42,43 In a group of 56 right-handed and 56 left-handed males, by using voxel-based morphometry, Herve et al42 could not find any differences in CST white matter between left- and right-handed subjects. Similarly, Westerhausen et al43 could not find any effect of handedness on CST in a DTI study in 30 left-handed and 30 right-handed subjects. A possible reason for the absence of any effect of handedness could be because handedness may depend on the pyramidal decussation and be related to the number of fibers passing to the other side, particularly fibers of the left CST, originating from the presumably dominant left hemisphere. Indeed, it has been found that more fibers cross from the left to right side than vice versa at the level of medulla oblongata.51 In fact, a higher number of CST fibers has been found in the right side of the spinal cord.52 Therefore, handedness would mostly affect the postdecussational segment of CST, rather than before decussation. Another plausible explanation could be that handedness is a cortical phenomenon and does not depend on the CST. In fact, a study found handedness related to asymmetry in the precentral cortex but not in the white matter along the course of CST.42 Or simply, handedness may be related to hemispheric dominance, which is left-sided even in most left-handed subjects. This would explain the left-sided asymmetry of CST white matter found by us and others.41–43
Methodologic Issues
There were several methodologic limitations in the present study. DTIs were acquired in 6 directions with 6 repetitions. In DTI, all data are ultimately fitted to obtain only the 6 tensor elements. Therefore, mathematically one needs only 6 directions to calculate the tensor and do further processing. However, such measurement does not have enough signal intensity–to-noise ratio (SNR). To enhance the SNR, one needs to acquire more data. For this, there are several options. One can repeat the 6-orientation measurement 6 times (ie, each direction is scanned 6 times, thus making a total of 36 acquisitions at each plane or section, which we did in this study), or one can acquire one 12-, 25-, 30-, or more orientation measurements within the given amount of time (ie, more number of directions or orientations is acquired, but only 1 time. Therefore, ultimately a total of only 12, 25, and ≥30 acquisitions are made at each plane or section). However in the latter case, though there are acquisitions in more directions, they are acquired only once, thus leading to a lower SNR for each direction compared with 6 directions × 6 repetitions.
Although increasing both the directions and repetitions will lead to better quality data, there are time and logistic constraints, particularly in a pediatric population. Therefore, there is always a trade-off between directions and repetitions. Also, purely from a mathematic point of view, we do not think 6 directions × 6 repetitions versus 12-, 25-, or 30-orientations × 1 repetition have that much difference in terms of overall SNR. However, it is true that the 6-orientation measurement has more orientation-dependent inhomogeneity in SNR. This means that certain orientations can be measured with higher SNR, whereas some orientations have poorer SNR. By adopting more orientations, such orientation-dependent SNR profile becomes more homogeneous (less orientation-dependent). In reality, within practical imaging time, this difference is hardly noticeable, particularly with well-oriented fiber bundles. Our study aim was to evaluate only the CST, which is a robust, well-defined, and thick fiber bundle, pretty much oriented in 1 direction. Therefore, we did not expect that our DTI acquisition protocol would have substantially affected the CST measurement because similar protocol across the subjects would have given the consistent results. Therefore, we believe that the data generated from 6 directions × 6 repetitions DTI should be appropriate and reasonable to achieve the goal of the present study, and also the consistency and homogeneity in data acquisition, throughout this study, made our results more meaningful and comparable.
Another issue of DTI deterministic tractography (used in the present study) is that even though it identifies the CST reliably, the uncertainty of the tract increases as the tracking algorithm approaches the low anisotropy areas such as motor cortex. Thus, precise characterization of the origin of tracts within small gyri may be difficult with DTI because the tract does not extend into the cortex. However, this may not be a major issue in the present study because we only attempted to localize the motor cortex between the pre- versus postcentral gyrus, and this does not require the tract to extend into the cortex itself within a gyrus. Moreover, the uncertainty of tracking is significantly smaller than the thickness of the gyri, and Hua et al53 have recently shown that there is a good concordance between the white matter fiber tracts including the CST, isolated by deterministic tractography, and the overlying cortex. They demonstrated that the isolated fiber tracts tend to end in the subcortical white matter regions of the corresponding cortical regions, known to be associated with that tract. Because of these factors, the localization of the motor cortex to the post- and precentral gyrus, by tracing the origin of CST, can be reliably performed by using DTI deterministic tractography. Similarly, DTI might be affected by the presence of other tracts in the isolated CST, thus resulting in decreased specificity. To minimize such contamination, we selected our seed region to include anterior descending fibers in the midpons region, where the contribution from other nonmotor tracts is negligible.17
The sensitivity of DTI could also be diminished by crossing and merging fibers, particularly as the tract descends into the internal capsule where the attenuation of white matter is quite high. This problem was reduced by reconstructing only the core of white matter tracts with known trajectories. Given that the CST is a large tract, we found that it was identifiable with DTI technique in almost all cases, suggesting that crossing fibers perhaps were not a major confounding factor. In a few cases in which we concluded that the CST was not identifiable, we could still isolate the CST, but it was not continuous from the brain stem to the primary motor cortex. Therefore, we considered the CST as unidentifiable and did not include these cases in further analyses.
Conclusions
Our study confirmed our hypothesis that the CST most frequently originates from both pre- and postcentral gyri, especially in older children, and was typically centered around two thirds of the distance from the anterior margin of the PLIC and occupied about 25% of its anteroposterior length. Children with CST originating from the precentral gyrus only are much younger than those with CST originating from the postcentral gyrus only or from both pre- and post- central gyri. Volume and FA of the CST also increases with age. However, sex or handedness appears to have no effect on the pattern of cortical origin of CST, its location on the PLIC, or its volume and diffusion properties.
Footnotes
Previously presented in part at: 62nd Annual Meeting of the American Epilepsy Society and 2nd Biennial North American Regional Epilepsy Congress, December 5–9, 2008; Seattle, Wash.
This work was supported by National Institutes of Health grants NS47550 (to E.A.) and NS34488 (to H.T.C.).
-
Indicates article with supplemental on-line tables.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received February 26, 2009.
- Accepted after revision May 20, 2009.
- Copyright © American Society of Neuroradiology