Abstract
BACKGROUND AND PURPOSE: Internal carotid artery (ICA) atheromatous disease is an important cause of ischemic stroke, and endarterectomy or stent placement is typically indicated for symptomatic patients with ≥70% stenosis. Our purpose was to compare contrast-enhanced MR angiography (CE-MRA) with unenhanced 2D time-of-flight MR angiography (2D TOF MRA) in detecting hemodynamically significant ICA stenosis, by using CT angiography (CTA) as the reference standard.
MATERIALS AND METHODS: This was an institutional review board−approved retrospective study. We identified 177 consecutive patients (354 ICAs) who received correlative CE-MRA, 2D TOF MRA, and CTA. Two neuroradiologists blinded to the CTA data graded the degree of ICA stenosis according to a 5-point scale. Additionally, luminal signal-intensity characteristics including 1) signal intensity drop-out, 2) distal-vessel narrowing, and 3) distal-vessel signal-intensity reduction were recorded. MRA results were correlated with those of CTA, and receiver-operating-characteristic (ROC) curves were constructed.
RESULTS: On CTA, there were 55 ICAs with and 299 without ≥70% stenosis. CE-MRA was 84% sensitive and 96% specific for detecting ≥70% stenosis; 2D TOF MRA was 80% sensitive and 95% specific. The area under the ROC curve was 0.97 for CE-MRA and 0.95 for 2D TOF MRA (P = .51, not significant). For both MRA studies, each of the luminal signal-intensity characteristics had high specificity (>98%) but poor-to-mild sensitivity (35%–66%) in detecting ≥70% stenosis.
CONCLUSIONS: Although it is established that CE-MRA more accurately delineates neurovascular anatomy than does unenhanced 2D TOF MRA, the administration of gadolinium did not offer a significant advantage in distinguishing surgically treatable ICA stenosis. This conclusion may be important in patients with contraindications to gadolinium.
Ischemic stroke is the third leading cause of death in Western countries, and carotid artery occlusive disease along with cardioembolic disease accounts for most of this morbidity and mortality. The need for medical or surgical treatment for symptomatic patients is determined on the basis of a ≥70% internal carotid artery (ICA) stenosis established by the North American Symptomatic Carotid Endarterectomy (NASCET) and European Carotid Surgery (ECST) Trials.1–3
Digital subtraction angiography (DSA) remains the reference standard of carotid artery luminal imaging and stenosis assessment.4–9 However, DSA has been shown to have a significant risk of mortality and morbidity, including a 4% risk of transient ischemic attack or minor stroke, a 1% risk of major stroke, and a <1% risk of death.10,11 In some very experienced centers, the rates of complications may be lower; however, DSA is still costly and time-consuming.12,13 For these reasons, many institutions including ours have replaced DSA with noninvasive techniques like duplex sonography (DUS), MR angiography (MRA), and CT angiography (CTA) in the presurgical evaluation of carotid artery disease. The literature reports excellent and robust sensitivities and specificities of DUS, MRA, and CTA in distinguishing <70% and >70% ICA stenosis.4,5,9,15–20 Nonetheless DSA is still used in more complex cases, for example, ones with multisegmental disease.
MR angiography is widely considered to be a safe, convenient, and noninvasive screening tool for carotid artery stenosis.7,9,17,20 Two-dimensional time-of-flight MR angiography (2D TOF MRA) is frequently used to evaluate carotid vasculature; however, it is vulnerable to signal-intensity drop-out artifacts in stenotic vascular segments and is typically suboptimal for evaluation of the great vessel origins.16,21,22 The addition of intravenous gadolinium for contrast-enhanced MRA (CE-MRA) helps to overcome these limitations; however, it also adds to the cost and complexity of imaging.14,16,23 Although CE-MRA clearly exceeds 2D TOF MRA in visualizing long-segment carotid artery morphology and anatomy,24 it does not necessarily follow that CE-MRA is superior for determination of a ≥70% ICA stenosis—the most important and well-studied imaging parameter in determining clinically relevant atheromatous disease. Moreover, given the recent concerns over nephrogenic systemic fibrosis (NSF), a significant proportion of patients, who are elderly, female, or have low glomerular filtration rates (GFR, <30–60), are increasingly being excluded from gadolinium administration (or receive a suboptimal dose).25,26 In such patients, unenhanced 2D TOF MRA could become a viable option for screening of the neck vasculature.
Our purpose, therefore, was to compare CE-MRA and unenhanced 2D TOF MRA in determining clinically significant ICA stenosis of ≥70%, by using CTA as a reference standard. CTA was chosen as the reference standard because of the following: 1) At our institution, DSA is no longer routinely performed as a confirmatory test for carotid artery stenosis; 2) CTA results correlate well with those of both DSA and pathologic samples; and 3) CTA has a stronger correlation with DSA than does DUS.4,5,10,18,19,27–37 CTA compared with MRA and DUS also has good spatial resolution, less flow dependence, and provides luminal and extraluminal data that augment evaluation of vascular narrowing.4,32–34,36–40 We hypothesized that unenhanced 2D TOF MRA provides sufficient and reliable vascular data to serve as a robust screening test in determining ≥70% narrowing. Additionally, we sought to assess luminal signal-intensity characteristics that contribute to the ICA stenosis assessment—specifically 1) signal-intensity drop-out at the point of maximal stenosis due to turbulent flow/intravoxel dephasing, 2) vessel narrowing distal to the lesion (“slim” sign), and 3) signal-intensity reduction distal to the lesion (a flow-dependent effect in MRA without gadolinium enhancement)—to evaluate the contribution to and correlation of each component with ≥70% degree of stenosis determination.
Materials and Methods
Patients
Our institutional review board, in agreement with the Health Insurance Portability and Accountability Act, approved this retrospective study protocol. We used an automated algorithm to search retrospectively a 3-year-period in our radiology data base for patients with CE-MRA, unenhanced 2D TOF MRA, and CTA imaging of the neck within 3 months of each other and with no interim carotid artery revascularization procedure. This search resulted in 190 consecutive cases. Due to incomplete study data, 13 cases were excluded (8 CE-MRAs, 4 2D TOF MRAs, and 1 CTA). The remaining 177 consecutive cases (or 354 carotid arteries) had CE-MRA, 2D TOF MRA, and CTA images of diagnostic quality (ie, no cases were excluded due to artifacts resulting from metal objects, swallowing/motion, and heavy circumferential calcification).
CE-MRA Protocol
CE-MRA was performed on a 1.5T Signa or LX system (GE Medical Systems, Milwaukee, Wis). Images were obtained by using a neck phased array coil; acquisition was oriented in the coronal plane. Contrast agent (Magnevist; Bayer Pharma, Wayne, NJ) was administered intravenously, 0.01 mmol/kg (or 0.2 mg/kg), at a rate of 3.0 mL/s as a single dose (∼20 mL); contrast bolus timing was 2 minutes. For the neck, timing and other parameters included TR of 20 ms, TE of 6 ms, flip angle of 45°, NEX of 1, and FOV of 28 × 18; the resulting voxel size was 1.25 × 1.25 × 1.6 mm.
Unenhanced 2D TOF MRA Protocol
MRA was performed on a 1.5T Signa or LX system (GE Medical Systems). Images were obtained by using a 2D TOF technique. For the neck, timing and other imaging parameters included TR of 24–27 ms, TE of 4.5–10.6 ms, flip angle of 60°, and NEX of 1 (ranges reflect minor protocol variations, representing worldwide practice). The typical image stack consisted of a set of contiguous 1.5-mm-thick axial images with 24 × 18 cm FOV and superior saturation, resulting in a voxel size of 1.25 × 1.25 × 1.6 mm.
CTA Protocol
CTA acquisition was performed according to standard departmental protocol on an 8- or 16-section multidetector CT (MDCT) scanner. The following parameters were used (minimal variations between scanners and sites are shown as ranges): start of scanning after 25-second delay (40 seconds for patients in atrial fibrillation) following nonionic contrast administration of iopamidol (Isovue 300; Bracco Diagnostics, Rome, Italy), 100–140 mL at 3 mL/s, via an 18-gauge intravenous power injector with saline push; 140 kilovolt (peak); 220–250 mA; 0.8- to 1.0-second rotation time; 2.5-mm section thickness reconstructed at 1.25-mm intervals; 3.75 mm/rotation table speed; and pitch of 0.75:1. Voxel size was 0.4 × 0.5 × 0.6 mm. Images were obtained from the C6 vertebral body level through the circle of Willis, followed immediately by a second set of images from the aortic arch to the skull base. The 2 sets of images, early phase and delayed phase datasets, were used to distinguish total ICA occlusion and hairline residual lumen.29 Afterward, source images were reconstructed into standardized maximum-intensity-projection (MIP) views of the intracranial and extracranial vasculature.
Image Analysis
MIPs from the CE-MRA and 2D TOF MRA and CTA MIP and axial source images were reviewed for evaluation of ICA narrowing, along with CE-MRA coronal and 2D TOF MRA axial source images (when at the discretion of the raters, the MIP data did not permit unequivocal assessment of stenosis). Each of 177 cases was reviewed by 1 of 2 experienced neuroradiologists. First, the CE-MRA images were presented in random order and rated over multiple reading sessions separated by days. A minimum of 2 weeks later, the 2D TOF MRA images were also presented in random order and rated during multiple readout sessions. Finally, following an additional delay of at least 3 weeks, ICA stenosis was graded on CTA images at separate readout sessions. While grading cases for any given technique, the readers did not have access to images of other techniques for the same case, were blinded to medical identifiers and clinical records, and were not allowed to consult with one another.
An Impax workstation (Agfa-Gevaert, Mortsel, Belgium) was used for retrieval and review of all cases. Percentage of ICA stenosis was graded according to the following 5-point scale: normal (score of 0), mild (score of 1, <50%), moderate (score of 2, 50%–<70%), severe (score of 3, 70%–<95%); and critical (score of 4, ≥95%), which is summarized in Table 1. All stenoses grading was based on visual interpretation of 2D TOF MRA, CE-MRA, and CTA; NASCET criteria measurements were not performed. The gray-scale level and window settings for MRA data ranged in width from 1350 to 1450 HU and in length from 600 to 700 HU. In addition, the presence or absence of 1) signal-intensity drop-out at the point of maximal stenosis, 2) distal-vessel narrowing, and 3) distal-vessel signal-intensity reduction beyond the point of the greatest narrowing was recorded for every vessel. A score of 1 indicated the presence of signal-intensity drop-out, distal narrowing, or distal signal-intensity reduction and consequently suggested a ≥70% ICA stenosis; whereas a score of zero indicated normal intraluminal signal intensity, absence of distal narrowing, or normal signal intensity in the distal-vessel and hinted at a <70% ICA stenosis.
Comparison of CTA versus CE-MRA, CTA versus 2D TOF MRA, and CE-MRA versus 2D TOF MRA stenosis scores
Intraobserver variability was assessed on the basis of rescoring of 20 (of 177, or 11.6% of the total) randomly selected duplicate cases that were inserted into the case queue of each reader. Interobserver variability was evaluated on the basis of rescoring of 36 (or 20.3% of the total) randomly selected cases including 18 that were previously scored as <70% ICA stenosis (scores of 0, 1, and 2) and another 18 that were previously scored as ≥70% (scores of 3 and 4).
Statistical Methods
For all statistical analysis, we used the Statistical Analysis System, Version 9.0 (SAS Institute, Cary, NC); in all cases, significance was rated as P < .05.
Compared with an ICA reference stenosis of ≥70% on CTA, receiver operating characteristic (ROC) curve analysis was performed; and sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) were calculated for CE-MRA and 2D TOF MRA.41,42 Additionally, the McNemar test was used to quantify whether the sensitivity and specificity of detecting ≥70% ICA stenosis by CE-MRA were significantly different from those of 2D TOF MRA (stenosis grades 0, 1, and 2 versus 3 and 4).
Again by using CTA as our reference standard, we examined the sensitivity, specificity, accuracy, PPV, and NPV of a positive finding (score of 1) of any of the 3 luminal signal-intensity characteristics associated with stenosis to predict severe and critical (ie, ≥70%) stenosis on 2D TOF MRA and CE-MRA.
Results
Demographics
Our patient population was composed of 87 women and 90 men with a mean age of 61.5 years (SD, 17.6 years; range, 20–96 years). In all 177 cases, CE-MRA and 2D TOF MRA were performed during the same imaging session, whereas CTA, on average, was completed within 12.6 days of the MRA studies (with 141 scannings performed within 5 days).
Stenosis Scores by Technique
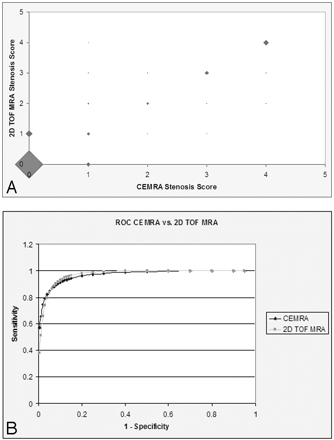
A sample carotid artery lesion with ≥70% stenosis visualized by CE-MRA, 2D TOF MRA, and CTA is shown in Fig 1A−E. Most carotid artery stenoses were graded as 0 (no stenosis) or 1 (mild) (270 on CE-MRA, 261 on 2D TOF MRA, and 277 on CTA); moderate (grade 2) stenosis was observed in 25 vessels on CE-MRA, 35 vessels on 2D TOF MRA, and 22 vessels on CTA; and severe (grade 3) or critical (grade 4) stenosis was found in 59 vessels on CE-MRA, 58 vessels on 2D TOF, and 55 vessels on CTA. Table 1 compares stenosis scores among all 3 imaging techniques: CTA versus CE-MRA, 2D TOF MRA versus CTA, and CE-MRA versus 2D TOF MRA. Ninety-five percent of stenosis scores on 1 technique were within 1 score category on another technique. A scatterplot of stenosis scores on CE-MRA and 2D TOF MRA is presented in Fig 2A.
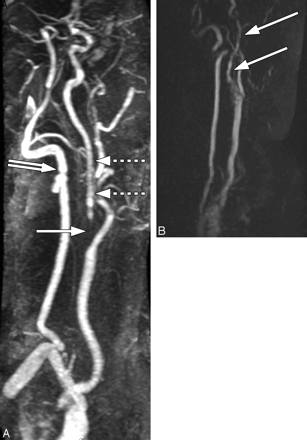
This ICA origin was rated by the observers to have severe (70%–95%) stenosis when imaged by all 3 techniques: 2D TOF MRA (A), CEMRA (B), and CTA (C–E). A and B, Signal-intensity drop-out is noted (arrow), but no distal narrowing or distal signal-intensity reduction is observed on MRA images. C, Curved reformatted CTA view of the left ICA demonstrating a severe stenosis (arrow). D, An axial image at the level of greatest narrowing (arrow). E, At the level of the NASCET reference diameter (arrow).
A scatterplot of stenosis scores on CE-MRA and 2D TOF MRA (A) and ROC curves for CE-MRA versus 2D TOF MRA (B). The size of the marker on the scatterplot represents the relative frequency of stenosis scores.
Test Characteristics by Technique
Sensitivity, specificity, accuracy, PPV, and NPV were calculated for CE-MRA and 2D TOF MRA in the detection of ICA stenosis of ≥70% (scores 3 and 4) by using CTA as the reference in determining the narrowing (Table 2). The area under the ROC curve for CE-MRA was 0.97, and the area under the curve for 2D TOF MRA was 0.95 (Fig 2B). There was no statistically significant difference between these 2 areas (P = .51). Similarly, the McNemar test showed no statistically significant difference between CE-MRA and 2D TOF MRA sensitivity (P = .68) and specificity (P = .99) in detecting ≥70% narrowing.
Sensitivity, specificity, accuracy, PPV, and NPV of CE-MRA and unenhanced 2D TOF MRA for detection of ≥70% ICA stenosis* based on CTA reference measurements
Intra/Interobserver Variability
In 20 random retest cases (40 carotid arteries) inserted to track intraobserver consistency, the first observer assigned the same score to 37 of 40 carotid arteries on both CE-MRA and 2D TOF MRA and a score that differed by 1 category (score of 1 versus 2) to 2 carotid vessels on 2D TOF MRA and another carotid vessel on CE-MRA. The second observer assigned the same score to 39 of 40 carotid arteries on CE-MRA and 2D TOF MRA and a score that differed by 1 category (score of 1 versus 2) to 1 carotid vessel on 2D TOF MRA.
In 18 cases that were previously scored as <70% ICA stenosis, readers reached an agreement in 33 of 36 carotid vessels on both CE-MRA and 2D TOF MRA and assigned scores that differed by 1 category (score of 3 versus 2) to 1 carotid vessel on CE-MRA and 2 other carotid vessels on 2D TOF MRA. In 18 cases that were previously scored as ≥70% ICA stenosis, readers reached an agreement in 34 of 36 carotid vessels on both CE-MRA and 2D TOF MRA and assigned scores that differed by 1 category (score of 3 versus 2) to 2 carotid vessels on CE-MRA.
Luminal Signal Characteristics
Most vessels (313/354 or 88%) did not show signal-intensity drop-out, distal-vessel narrowing, or distal-vessel signal-intensity reduction on either of the MRA sequences. Fig 1A, -B and Fig 3A, -B demonstrate signal-intensity drop-out, distal-vessel narrowing, and distal-vessel signal-intensity decrease. Signal-intensity drop-out on 2D TOF MRA corresponded to ≥70% stenosis on CTA in 88% (28/32) of cases, and signal-intensity drop-out on CE-MRA corresponded to ≥70% stenosis on CTA in 88% (36/41) of cases. Both distal-vessel narrowing and distal-vessel signal-intensity reduction were found infrequently (25 ICAs or 7.1% by CE-MRA, 22 ICAs or 6.2% by 2D TOF MRA, 25 ICAs or 7.1% by CE-MRA, and 23 ICAs or 6.5% by 2D TOF MRA, respectively). Each of the 3 luminal signal-intensity characteristics associated with stenosis, for both CE-MRA and 2D TOF MRA, had a good specificity but poor sensitivity in detecting surgically relevant stenosis of ≥70% (Table 3).
A, MRA of the neck with gadolinium demonstrating signal-intensity drop-out in the proximal left ICA (solid arrow), with decreased signal intensity and vessel narrowing (slim sign) in the distal ICA (dashed arrows), which is significantly smaller compared with the ipsilateral vertebral artery (double-tailed arrow). B, 2D TOF MRA image of the neck vasculature exemplifies distal-vessel narrowing/irregularity and distal-vessel signal-intensity reduction (white arrows).
Sensitivity, specificity, accuracy, PPV, and NPV of signal drop-out, distal-vessel narrowing, and distal-vessel signal-intensity reduction for detection of ≥70% ICA stenosis* based on CTA reference measurements
Discussion
We have shown that with MRA there is no significant added benefit from gadolinium administration in determining ≥70% ICA stenosis when CE-MRA is compared with 2D TOF MRA (P = .51) by using CTA as reference standard. In our study population, CE-MRA had a sensitivity of 84% and a specificity of 96%, whereas 2D TOF MRA had a sensitivity of 80% and a specificity of a 95%. Additionally, each of the 3 luminal signal-intensity characteristics contributing to ICA stenosis assessment exhibited excellent specificity (>98%) but only moderate sensitivity (35%–66%) in detecting ≥70% ICA narrowing. Such results suggest that the presence of signal-intensity drop-out, distal narrowing, and distal signal-intensity reduction is associated with ≥70% ICA stenosis, whereas the absence of signal-intensity drop-out, distal narrowing, and distal signal-intensity reduction does not necessarily correlate with <70% ICA narrowing.
On the basis of NASCET and ECST data, in a clinical setting, one must assess which of the available imaging tools is sufficiently accurate to distinguish patients with severe and critical stenosis (≥70%) from those with moderate, mild, or no narrowing.1–3 Our data show that unenhanced 2D TOF MRA is sufficiently accurate to identify patients with ≥70% ICA stenosis compared with CE-MRA.
In the setting of screening for surgical ICA disease, where the obligation is to not miss disease, both CE-MRA and unenhanced MRA are known to be sensitive but not necessarily specific. Our results, based on a sample of 177 consecutive patients that reflected a clinically typical case mix of ICA stenoses, show that the unenhanced 2D TOF MRA is nondistinguishable from CE-MRA as such a screening test. The use of unenhanced 2D TOF MRA in screening for severe ICA disease may be particularly important in select clinical settings. For example, in the emergency department when a patient with stroke comes in over the weekend (when no DUS technician is available) and diffusion-weighted imaging is being performed, often it is clinically important to know whether there is ≥70% ICA stenosis.
Our findings may not only translate into cost reduction by questioning the need for gadolinium administration in certain populations but could also benefit patients with renal insufficiency who may be harmed by the recently described NSF.26 Although the exact pathophysiology of NSF is unclear, available reports suggest that NSF occurs due to a combination of decreased kidney function, presence of inflammation, and exposure to gadolinium-based contrast agents.25,26 As an increasingly larger portion of elderly patients with decreased GFR are excluded from gadolinium administration due to concern over NSF, 2D TOF MRA can serve as an accurate and powerful tool for evaluation of carotid artery stenosis while improving the safety profile of clinical practice.
Nonetheless, in certain clinical settings administration of gadolinium will remain invaluable because CE-MRA provides more robust morphologic and anatomic vascular data that might be particularly important in preparation for surgery or stent placement.16,24 Most important, CE-MRA performs better than 2D TOF MRA in the visualization of such findings as hairline occlusion, dissection, or tandem lesions, detection of which can have important implications for patient management.20,23
One of the shortcomings of 2D TOF MRA is vulnerability to signal-intensity drop-out (or flow-void) artifacts resulting from intravoxel dephasing related to turbulent flow associated with the stenotic segments of vasculature.21,22 The literature suggests that the signal-intensity drop-out artifacts are found in approximately 10%–20% of all 2D TOF MRA studies and that their presence is highly correlated with severe ICA stenosis (70%–99%).22 Nederkoorn et al22 reported that 84.3% of signal-intensity drop-out corresponds to severe stenosis when confirmed by DSA. In our study, signal-intensity drop-out was present in 9.0% (32/354) of 2D TOF MRA studies, and its presence correlated with a ≥70% stenosis found on CTA in 88% (28/32) of the patients.
There are a number of potential limitations of our study. One is the use of CTA rather than DUS or DSA as a reference standard for carotid stenosis measurement. The literature often characterizes DUS data as operator-dependent and cautions against using DUS as the sole perioperative imaging study.9,43 Although there is still ongoing controversy over whether the results from CTA match completely with selective intra-arterial angiography or rotational angiography,44,45 CTA has been favorably evaluated against DSA in ICA stenosis assessment,4,18,19,29,30 with some patient series achieving CTA sensitivity and specificity for detecting ≥70% carotid artery stenosis in the range of 98%–100%.4,19,27,29,30 Also, in a direct comparison of CTA with DUS by using DSA as a reference standard, CTA showed a better correlation coefficient, sensitivity, and specificity in evaluating ICA stenosis than did DUS.30
Randoux et al4 assessed gadolinium-enhanced MRA and CTA against DSA in detecting severe ICA narrowing, and CTA showed a better correlation with DSA across all stenosis values compared with MRA. In that study, CTA was 100% sensitive and 100% specific in detecting ≥70% ICA stenosis, whereas MRA was 93% sensitive and 100% specific and also was found to overestimate the extent of vessel narrowing.
Overestimation of carotid artery narrowing on CE-MRA can result from the dephasing artifacts along the margin of the lumen (which become exaggerated in areas of tight narrowing), the signal-intensity threshold used to create the MIPs, and the section thickness causing partial volume averaging effect.4,46,47 CT angiography is less prone to overestimating carotid artery stenosis because it is an anatomically weighted vascular imaging technique with a higher spatial resolution due to thinner sections, with pixel size being typically <0.4 mm on MDCT.19,38,48 This is in contrast to gadolinium-enhanced MRA, which has lower resolution, with a pixel size typically approximately 1 mm, and which is physiologically weighted and therefore subject to pulsation and turbulence artifacts. Additionally, CTA is capable of distinguishing total ICA occlusion from hairline lumen, which has an important prognostic value for patients being considered for endarterectomy.29,33,49
Moreover, with the ongoing improvements in CT technology and the development of MDCT, CTA has become even more reliable, reproducible, and accurate in evaluation of ICA stenosis.31–33,36,37 MDCT angiography has been shown to provide vascular and tissue data with unprecedented detail and spatial resolution, recently permitting a direct measurement of carotid artery stenosis at the point of the greatest narrowing.32,33,38 Also, in recent studies by Saba et al35 and de Weert et al,39 MDCT angiography showed excellent correlation with pathologic specimens in quantification of total plaque area, calcified area, fibrous tissue area, and lipid core area in mildly calcified plaques and in the detection of carotid plaque ulcerations. The accuracy of MDCT angiography, however, can be dependent on the postprocessing methodology. In our study, we confirmed the CTA MIP data with axial source images, an approach that has been proved optimal.50,51
Given all of these characteristics of CTA, the strength of the published CTA literature, and the unavailability of confirmatory DSA in our study cohort, we felt justified in selecting CTA as our de facto reference standard for degree of ICA narrowing.
There are other limitations to our study design, such as the pooling of 2D TOF MRA studies with different TE values (4.5- to 10.6-ms range). The literature suggests that shorter TEs reduce the occurrence of signal-intensity drop-out and consequently can affect interpretation of the extent of focal stenosis if signal-intensity drop-out is used as a marker of ≥70% ICA narrowing.22,52,53 However, on the basis of published work and our results, it is clear that our TEs were sufficiently long to permit signal-intensity drop-out from intravoxel dephasing.53 Conversely, 2D TOF MRA with long TEs bears the risk of increased occurrence of signal-intensity drop-out, which could result in overestimation of ICA stenosis. This limitation becomes important in highly stenotic vessels when one attempts to substratify severe carotid artery stenosis (eg, to differentiate 80% and 90% of narrowing), whereas the objective of this study was to specifically distinguish <70% and >70% ICA stenosis.22,53 Yet another potential limitation was due to the difficulty in distinguishing calcification from contrast agent on CTA in vessels with heavy circumferential calcified plaque.4,6,20,54 Nonetheless, in our study population, we did not encounter circumferential calcification severe enough to limit our ability to grade carotid stenosis accurately.
As a part of our CTA protocol, we used a fixed scanning delay following contrast administration. Currently available techniques such as a test bolus or bolus tracking are more appropriate because they optimize arterial enhancement and reduce the volume of contrast agent injected. Also, our reference standard, CTA with a section thickness of 2.5 mm reconstructed with 50% overlap, is no longer state of the art. Although it is an imperfect standard, it was used as a common denominator to compare 2 techniques. Our goal was not to calculate absolute values of CE-MRA and 2D TOF MRA sensitivity and specificity but rather to assess their equivalence or lack of equivalence. Similar to comparable published reports, we included the contralateral carotid arteries (likely ones with no or low-grade stenosis) in our sensitivity and specificity analysis.31 This might have resulted in under-representation of the stenotic vessels and overestimation of the specificity of CE-MRA and 2D TOF MRA and should be considered a limitation.31 Additionally, in our study we relied on unenhanced 2D TOF MRA; and unenhanced 3D TOF MRA may be more accurate than 2D TOF MRA.55
Conclusions
Our study focused on a narrowly defined application of CE-MRA, specifically, screening and identification of patients with ≥70% ICA stenosis. Although it is clear that CE-MRA more accurately delineates neurovascular anatomy than does unenhanced 2D TOF MRA, the administration of gadolinium did not offer significant advantage in distinguishing surgically treatable ≥70% ICA stenosis in the patient cohort we studied. This conclusion may be important in patients with contraindications to gadolinium administration, especially for the elderly patients with reduced GFR at risk for NSF.
References
- Received September 18, 2008.
- Accepted after revision November 18, 2008.
- Copyright © American Society of Neuroradiology