Abstract
BACKGROUND AND PURPOSE: Early evaluation of the pyramidal tract is a prerequisite in patients with intracerebral hemorrhage (ICH) in order to decide the optimal treatment or to assess appropriate rehabilitation. The aim of this study was to evaluate and predict the neuromotor and functional outcome of an ICH by using diffusion tensor imaging (DTI) in the acute phase.
MATERIALS AND METHODS: Eighteen patients with a hemiparetic supratentorial ICH were prospectively studied with DTI within 2 days after onset. A region-of-interest-based analysis was performed for the fractional anisotropy (FA) of the pyramidal tract in the cerebral peduncles. The degree of paresis was assessed at day 0 and day 28 by paresis grading (PG). The functional outcome was evaluated by the modified Rankin Scale (mRS).
RESULTS: The FA in the affected side was significantly lower compared with that of the unaffected side (P = .001) with the mean diffusivity remaining unchanged (P = .50). The ratio of the FA (rFA) in the affected side to the unaffected side was significantly correlated with the PG at day 0 and 28 and the mRS score at day 28 (P = .002, r = −0.674; P < .001, r = −0.767; and P = .002, r = −0.676). The rFA for the good and poor outcomes based on the PG was significantly different (P < .001). The cutoff point of the rFA for the good and poor outcomes was set at 0.85 (sensitivity, 100%, specificity, 100%).
CONCLUSIONS: We conclude that DTI can evaluate the motor deficit quantitatively and may predict the functional outcome in patients with an ICH who were scanned within 2 days after the ICH onset.
Predicting the neuromotor outcome of patients with an intracerebral hemorrhage (ICH) in the acute stage would provide us with new information to determine the best therapy or to design an appropriate rehabilitation program. The preservation or recovery of the pyramidal tract is important for a good recovery of the impaired motor function in patients with stroke.1 Several authors have tried to predict the functional and/or motor outcomes by using MR imaging. The presence of wallerian degeneration in the pyramidal tract remote from the initial lesion on MR images correlates with a poor outcome,2,3 but the signal-intensity changes are generally not detected until 4 weeks after a stroke by using conventional MR imaging techniques.4
It has recently been reported that diffusion tensor imaging (DTI) can detect a signal-intensity change in the ipsilateral pyramidal tract in patients with an ischemic stroke earlier than conventional MR imaging techniques.5,6 The decrease in the fractional anisotropy (FA) in the involved pyramidal tract becomes progressively reduced during the subacute-to-chronic stages of an ischemic stroke7–9 and correlates with the motor deficit.7,10–12 With regard to hemorrhagic diseases, a few studies have reported that a change in the FA correlates with the functional outcome, using DTI or diffusion tensor tractography.13–15 However, the MR images were obtained at least 3 days after the onset or hematoma removal in these studies, and it is unclear how the FA changes in the very acute phase of ICH or whether the change in the FA in the very acute phase of an ICH correlates with the functional outcome or the motor outcome.
The aim of this study was to quantify and predict the neuromotor outcome of patients with ICH by using DTI in the acute phase within 2 days after the onset.
Materials and Methods
Patient Selection
In total, 18 patients were recruited for this study (11 men, 7 women). The inclusion criteria were as follows: 1) a supratentorial ICH diagnosed by a CT scan; 2) motor deficit on admission; 3) no previous history of stroke or brain injury on MR imaging, or other neurologic disease; 4) conservative treatment without a surgical intervention; and 5) the DTI performed within 2 days of the onset. All the patients started rehabilitation for motor weakness within 3 days after the onset. The study was approved by the local ethics committee. An informed consent was provided by all patients.
Clinical Assessment
The neurologic deficit was evaluated on the basis of the severity of motor paresis, which was estimated at day 0 and day 28, and the functional outcome was assessed by the Modified Rankin Scale (mRS)16 and the Barthel Index17 on day 28. The motor performances of the upper and lower extremities were evaluated by the modified National Institutes of Health Stroke Scale (NIHSS).18 The estimated values for the upper and lower extremities of the NIHSS were summed and defined with paresis grading (PG) ranging from 0, no motor weakness, to 8, hemiplegia (Table 1). All of the patients were divided into good and poor groups depending on the PG on day 28. Patients with a PG of 0–2 were graded as good, and those with a PG of 3–8 were graded as poor.
Scale definition of PG*
MR Imaging
The MR imaging was performed with a 3T superconducting magnet (Signa; GE Healthcare, Milwaukee, Wis). The DTI data were acquired by using a single-shot echo-planar imaging sequence (TR, 10000 ms; TE, 75 ms; NEX, 2; matrix size, 128 × 128; FOV, 260 mm; voxel size, 2.0 × 1.4 × 4.0 mm). The brain was imaged with a section thickness of 4 mm and no intersection gap, with 36 standard axial images. The diffusion-weighting was encoded along 6 independent orientations, and the b-value was 1000 s/mm2. An 8-channel phased array head coil was used. The total imaging time was approximately 2 minutes 40 seconds.
We performed the following preliminary studies with 3 healthy volunteers (ranging from 24 to 48 years of age):
Left-Right FA Symmetry Test
The DTI study was performed by using the same protocol as that used in patients enrolled to this study. The FA was measured and compared in both of the cerebral peduncles.
Motion-Probing Gradient Test
We also evaluated the differences in the quality of the DTI images, the FA, and the acquisition time under different motion-probing gradient (MPG) settings and determined the suitable MPG for this study.
Data Processing
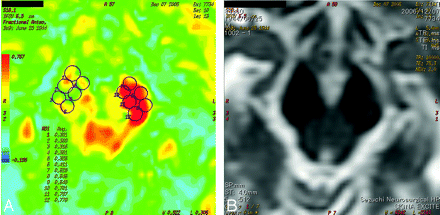
The DTI datasets were transferred to a workstation and were processed by using SPM99 in Matlab 5.3 (MathWorks, Natick, Mass). Six small regions of interest were manually drawn in the anterior part of the bilateral cerebral peduncles on the 2D FA color map on the basis of the T2-weighted image and anatomic knowledge (Fig 1). The FA and mean diffusivity (MD) of the regions of interest were averaged. The average values of the FA and MD were compared in the affected and unaffected sides. Additionally, the ratio of the FA (rFA) between the affected side and the unaffected side was calculated (eg, rFA = FAAffected Side / FAUnaffected Side).
The FA color map (A) and T2-weighted image (B) of patient 17 obtained at day 0. Six small regions of interest (ROIs) are manually placed on the bilateral cerebral peduncles on the color FA map (A) on the basis of the T2-weighted image (B) and anatomic knowledge. The FA of each region of interest was averaged. Note that there is not a significant signal-intensity change in the right cerebral peduncle on the T2-weighted image; however, the FA is markedly reduced in the right side. The motor outcome of this patient is poor. Avg. indicates average.
Statistical Analysis
All values were presented as the mean ± SD. A group comparison was made by using the Wilcoxon rank sum test. The Spearman rank correlation coefficient was calculated for comparison of the rFA and the clinical scores. To ascertain the cutoff point of the rFA that classified the outcome of good and poor, we used a likelihood ratio χ2. The 5 variables (ie, age ≥80 years; a Glasgow Coma Scale (GCS) score ≥12 points; an ICH volume ≥20 mL; narrow pulse pressure, <60 mm Hg19,20; and the rFA ≥ 0.85) were used to determine the correlation with the motor outcome by using a Spearman rank correlation coefficient. JMP 5.1 (SAS Institute, Cary, NC) was used for the analysis. A P value of <.05 was used to indicate statistically significant differences.
Results
In healthy volunteers, the FA in the bilateral cerebral peduncles was 0.79 ± 0.09 in the right and 0.80 ± 0.02 in the left at 6 MPG and 0.81 ± 0.03 in the right and 0.80 ± 0.04 in the left at 13 MPG. There were no significant differences in the cerebral peduncles at both 8 MPG (P = .85) and 13 MPG (P = .75). There were also no significant differences in the FA between 6 MPG and 13 MPG in each side of the cerebral peduncle (right, P = .79; left, P = .86). On the other hand, for the acquisition times of the different MPG directions, it took 2 minutes 20 seconds and 4 minutes 40 seconds to obtain DTI images, respectively. This difference of >2 minutes is long for patients with a very acute phase of ICH. Therefore, 6 MPG was used for the DTI in this study.
The clinical features of patients are shown in Table 2. The 18 patients (11 men, 7 women) with acute supratentorial ICH ranged in age from 30 to 99 years (67.8 ± 16.2 years), and the motor weaknesses were studied. The lesion locations were in the putamen in 7, the thalamus in 6, and the subcortex in 5 patients. DTI was performed within 2 days after the onset (0.72 ± 0.89 days). The motor function at day 28 improved in 15 patients, was unchanged in 2, and deteriorated in 1 patient compared with the motor function at day 0.
Summary of clinical data in 18 patients
Table 3 shows the FA and MD diffusion parameters in the cerebral peduncle of the affected and unaffected sides. The FA significantly decreased in the affected side (0.60 ± 0.063 versus 0.68 ± 0.046, P = .001), whereas the MD did not show a significant difference (P = .50). In Table 4, the correlations between the rFA and the clinical scores are shown. The rFA was negatively correlated with the PG at day 0 (P = .002, r = −0.674) and at day 28 (P < .001, r = −0.767). In addition, the rFA had a negative correlation with the mRS score measured at day 28 (P = .002, r = −0.676). On the other hand, the Barthel Index was not significantly correlated with the rFA (Table 4). The mean values of the GCS scores on admission, the hematoma volume, and the pulse pressure were 12 ± 2.07 points, 16.1 ± 12.1 mL, and 76.2 ± 19.0 mm Hg, respectively. Among the 5 variables, only the rFA correlated significantly with the motor outcome defined as good and poor (Table 5).
Region-of-interest analysis*
Statistical analysis between FA ratio and clinical scores*
Relationship between the motor outcome and various parameters*
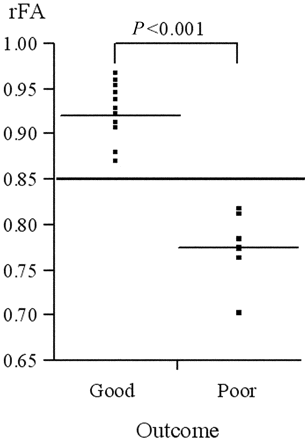
The rFA of the good and poor groups was plotted (Fig 2). There was a significant difference between the mean values of the 2 groups (P < .001). The cutoff point of the rFA that divides the 2 groups was set at 0.85 (sensitivity, 100%; specificity, 100%). The FA color map and T2-weighted image of patient 17 are shown in Fig 1. These images were obtained at day 0, and the FA was markedly reduced in the right cerebral peduncle. The motor outcome of this patient was poor at day 28.
The patients are divided into good and poor on the basis of the score of the PG evaluated at the 28th day, and the ratio of the FA is plotted. The patients with 2 or <2 are graded as good, and those with ≥3 are graded as poor. There is a significant difference between the mean values of the 2 groups (P < .001). The cutoff point of the rFA that divides the 2 groups is set at 0.85.
Discussion
The present study was designed to evaluate whether the functional outcome in patients with an acute-phase ICH can be predicted by measuring the FA in the cerebral peduncles within 2 days after onset. The FA measured in the affected cerebral peduncle decreased by 11% compared with the FA measured in the unaffected side. The rFA was negatively correlated with the PG both at day 0 and 28 days after onset and with the mRS score 28 days after the onset. The rFA showed a significant difference between the good and poor groups. Furthermore, only the rFA correlated significantly with the motor outcome among the variables that were used in the ICH scales. We conclude that DTI was able to predict the neuromotor outcome and may predict the functional outcome of the patients with ICH by measuring the FA in the acute phase of an ICH.
In previous studies, the neuromotor outcome was found to correlate with conventional neuroimaging findings such as the size, the volume,11,21 and the extent of the infarction.22,23 More recent studies reported the correlation between the reduction of the FA and the motor function in patients with a subacute or chronic phase ischemic stroke.11,22,23 For patients with ICH, only a few studies using DTI have been conducted to evaluate the neurofunctional outcome.2,14,15,24 Yokoyama et al15 reported that the mean FA ratio in the affected pyramidal tract decreased 11% compared with that in the unaffected side among the good-recovery group. In contrast, the mean FA decreased 23% among the poor-recovery group. Kuzu and Inoue13 used the absolute value of the FA, and there was a 9% difference in the FA between the good and poor patient groups. In the present study, the rFA correlated significantly with the PG at both day 0 and day 28. This means that the FA measured in the very early phase of an ICH may predict the motor outcome. The rFA correlated only with the mRS, but not with the Barthel Index. The mRS is considered as a global disability scale with 6 different grades, and the Barthel Index has more indices than the mRS and concentrates on wider activities of daily living than the mRS.25,26
Some parameters used in the ICH scales19,20 were applied to our patients; however, none of them correlated with the outcome of good or poor. We limited the outcome to only the motor deficit of the extremities in patients with a small-to-moderate amount of hematoma. On the other hand, the outcome scales highlighted the mortality rate or the general outcome. The motor deficit largely affects the functional outcome; however, the existence of aphasia, neglect, and ataxia should be considered to determine the patient's outcome. That might explain why the rFA did not correlate with the Barthel Index in the present study or why these parameters did not have a significant correlation with the outcome of our patient group. We defined the cutoff point of the rFA that classified the motor outcome as good and poor to be 0.85. The mean value of the rFA for each group was significantly different. Measuring the FA in the very early phase of an ICH would be beneficial for patients to determine the best therapy or to design a suitable rehabilitation program. Furthermore, it might be helpful in deciding the surgical indication.
Diffusion anisotropy mirrors the fiber integrity and the degree of the fiber organization in the white matter tract.11,23 Axonal loss, gliosis, and an accompanying increase in the extracelluar matrix have been generally considered to be the determinants of the decrease in diffusion anisotropy in wallerian degeneration.10 Diagnosing wallerian degeneration requires at least 4–5 weeks after the stroke when using T2-weighted images, the apparent diffusion coefficient, or other conventional MR imaging parameters, whereas the FA is more sensitive than these conventional MR imaging parameters for detecting wallerian degeneration.10,11
Several DTI studies have reported a reduction of the FA with an unchanged MD on the affected side in the pyramidal tract distal to the infarct foci after an ischemic stroke.10,11,13,23 They reported that the FA in the affected pyramidal tract was reduced 13%–32% during the subacute-to-chronic phase of an ischemic stroke, and the reductions in the FA were more severe at later time points of 2 days to 1 year.10,11,12,29 Yoshida et at14 reported that the FA ratio in the affected side decreased 20% within 5 days after the onset. In the present study, the FA decreased by 11% in the affected cerebral peduncle with an unchanged MD within 2 days after the onset of an ICH. This finding is consistent with results from previous studies. Axonal transport in the neuronal axons is disturbed by a direct injury or compression by an ICH. The loss of axonal structures, membrane disintegration, and cellular debris then lead to a decrease in the first eigenvalue and a relative elevation of the second and third diffusion eigenvalues. Consequently, this phenomenon results in a reduced anisotropy, whereas the effect on the orientationally averaged diffusion index is difficult to predict and the MD may remain unchanged in the acute phase of an ICH.
We defined the regions of interest for the FA measurement in the cerebellar peduncles distal to the primary lesion on the basis of anatomic knowledge28; however, we cannot eliminate the arbitrariness entirely. Furthermore, the FA is accurate at a single fiber and is affected by the crossing fibers because of the partial volume effect in the voxel. This phenomenon is known as the pseudodiffusion anisotropy decrease,14 which may lead to the underestimation of anisotropy in the lesion. The pyramidal tract usually represents both the corticospinal tract and the corticobulbar tract.15 The corticospinal tract is a large and highly anisotropic tract; on the other hand, the corticobulbar tract is easily affected by other fibers running in the anteroposterior projection.29 Moreover, the pyramidal tract does not run precisely perpendicular to the axial plane. Therefore, the FA in the cerebral peduncle may not exactly reflect the pyramidal tract. Diffusion spectrum imaging30 or Q-ball imaging,31 which have recently been proposed, might overcome this issue32 but are still far from clinical use because of their long acquisition times and data analyses.29,33 We used the FA ratio and not the absolute value of the FA to reduce this crossing-fiber problem.
The number of MPG is one of the unresolved issues in the DTI data acquisition. Six MPG directions were used in this study. The anisotropy errors at 6 MPG were higher than those at higher MPGs. However, the error was found to be approximately 3 times smaller than that in the relative anisotropy.34 There was no significant difference in the FA from 6 and 13 MPG in the cerebral peduncles according to our preliminary study. The 6-MPG acquisition is more tolerable to the patients, given the very acute phase of the ICH. Our method might contain some problems; however, we believe that it still has an advantage for evaluating the patients in a neurosurgical emergency.
There are certain limitations to this study. First, the patient population was small. Therefore, the interpretation of the results of this study should be done with caution. Second, patients treated conservatively with a relatively small hematoma were enrolled. Patients treated surgically should be enrolled, and the FA at pre- and postsurgery should be examined in a future study. This may give us information regarding the indications for surgery.
Conclusions
We demonstrated that DTI can evaluate the motor deficit quantitatively and may predict the functional outcome in patients with an ICH who were scanned within 2 days after the onset. This study detects an early change in the affected pyramidal tract at the level of the cerebral peduncle. DTI can offer us important information and can be used to predict the motor outcome of the affected extremities in hemiparetic patients with an ICH.
Acknowledgments
We are deeply grateful to Seguchi Neurosurgical Hospital stroke care team whose enormous support was invaluable during the course of our study. We also would like to thank Tsuyoshi Tada, MD, Medical Education Center, Shinshu University School of Medicine, and Nunung NurRahmah, MD, Shinshu University Graduate School of Medicine, for their advice on this study.
References
- Received January 8, 2009.
- Accepted after revision March 23, 2009.
- Copyright © American Society of Neuroradiology