Abstract
BACKGROUND AND PURPOSE: Radiation-induced cognitive dysfunction is a common and serious complication after radiation therapy of brain tumor, yet knowledge of its mechanism is poorly understood. The aim of this study was to establish a young rat model for acute radiation encephalopathy, at both cognitive and pathologic levels, induced by fractionated irradiation.
MATERIALS AND METHODS: Four-week-old male rats were randomized into sham (0 Gy) and 2 experimental groups receiving fractionated irradiation of 5 Gy/day, 5 days/week, with total doses of 20 and 40 Gy, respectively. Cognition, BBB integrity, and potential astrogliosis were evaluated at 0, 4, 8, and 12 weeks' postirradiation.
RESULTS: Twenty-Gy irradiation led to transient cognitive impairment only at 4 weeks' postirradiation. Forty-Gy irradiation induced cognitive impairment at both 4 and 8 weeks' postirradiation, which was more severe than that induced by 20 Gy. Cognitive impairment was accompanied by a transient increase in BWC only at 4 weeks for the 40-Gy group. Disrupted BBB permeability was detected at 4 and 8 weeks' postirradiation for the 20-Gy group, and at 4, 8, and 12 weeks' postirradiation for 40-Gy group, respectively. Increased astrogliosis in the hippocampus could be detected at 4 weeks' postirradiation for 40-Gy group.
CONCLUSIONS: Fractionated irradiation in this experiment could induce acute brain injury, leading to cognitive impairment in young rats. BBB disruption might be a sensitive index for acute radiation encephalopathy. In addition, reactive astrogliosis might play an important role in this process. The present model, especially the 40-Gy irradiation group, is useful for basic and therapeutic studies of acute radiation encephalopathy.
Abbreviations
- BBB
- blood-brain barrier
- BWC
- brain-water content
- EB
- Evans blue
- GFAP
- glial fibrillary acidic protein
- IF
- immunofluorescent
- NPC
- nasopharyngeal carcinoma
- RE
- radiation encephalopathy
Radiation therapy is used widely for treatment of diffuse primary and metastatic brain tumors,1,2 and its curative efficacy is well-established. With improvement of patient survival, attention is paid to its adverse effects, the radiation-induced brain injury.
In Guangdong province of southeastern China, NPC constitutes approximately 32% of all cancers. The incidence of overall NPC in Guangdong province is >20 per 100,000 people every year among men and it is the most common kind of cancer in the province.3 The rate in Cantonese speakers is double that in other dialect groups, such as Hakka, Hokkien, and Chiu Chau,4 and is, to the best of our knowledge, the highest in the world.5 For patients with NPC, radiation therapy is the first-choice treatment and sometimes the only effective management of the disease.
For the treatment of patients with NPC, the radiation fields cover both the face and neck regions, which inevitably subject the bilateral inferior temporal lobes to the radiation exposure. As a consequence, radiation-induced brain injury may occur hours, days, weeks, months, and even years after exposure to radiation, which is known as acute and chronic RE. RE following radiation therapy for NPC was reported to occur in 0.9%- 4% of individuals,6 in whom elevated intracranial pressure, caused mainly by cerebral edema, and progressive deterioration of hippocampal-associated learning and memory functions are perhaps the most common sequelae of the radiation therapy.7,8 This can be especially devastating to patients and caregivers.
Appropriate models are needed to integrate the complex influences that occur in the radiation-exposed individual, to develop approaches for prophylaxis, mitigation, and treatment for RE. In this regard, an early-onset young rat model of radiation-induced cerebral injury following single-dose exposure has been previously established.9 Nevertheless, with the aid of a stereotactic technique, fractionated radiation therapy has been demonstrated to have longer survival and fewer complications compared with single-dose radiation therapy.10
To establish an animal model that closely resembles the most frequently used clinical protocol for cranial irradiation so as to better understand the underlying mechanisms and the clinical manifestation, we exposed young rats to fractionated irradiation treatments by using a linear accelerator and evaluated the changes in cognition, BBB permeability, and histopathology.
Materials and Methods
Animal
A total of 144 male Sprague-Dawley rats, 4 weeks of age and weighing 110 ± 20 g, were used in this study. Rats were housed socially in a temperature-controlled room with free access to food and water. Animal care and the experimental procedures in this study were approved by the Animal Care and Ethics Committee at Sun Yat-sen University, China.
Protocol of Irradiation
Rats were randomized into 3 groups (n = 48) to receive different irradiation treatments by a Linear accelerator (PRIMUS Linear Accelerator; Siemens, Erlangen, Germany) at room temperature. Animals were anesthetized with pentobarbital (30 mg/kg, peritoneal injection) and were restrained in the sternal recumbent position on a treatment table. The head was centered in the exposure field with eyes, mouth, neck, and body protected with customized lead shielding. For each experimental group of the rats, 5-Gy (dose rate, 300-cGy/min) irradiation was given per day, 5 days/week, until the total dose reached 20 and 40 Gy, respectively. Rats in the sham control group received 0 Gy of irradiation but underwent the same stress as did the others.
The 48 rats in each treatment group were further randomized into 4 time groups, with 12 rats in each time subgroup, for follow-up cognitive tests, histopathologic examinations, and evaluation of their BBB permeability and BWC at 4 different time points (0, 4, 8, and 12 weeks' postirradiation). The time point of zero was the first day after the completion of the irradiation.
General Observations and Body Weight
Following the treatment, rats were housed under identical experimental conditions to observe their feeding and drinking behavior, limb movements, and local skin reaction to the irradiation. Their body weights were monitored weekly. All these observations were recorded and used as indexes of potential changes following the irradiation.
Morris Water Maze
Potential cognitive changes of the rats were evaluated by using the Morris water maze test, which was performed chiefly according to previously reported procedures.9,11 In brief, a circular water tank (120 cm in diameter, 40 cm in depth) filled with water at 26 ± 2°C was divided into 4 quadrants, with a platform placed in the center of 1 of the 4 quadrants in the pool. A video camera and monitor over the pool were used for automatic video recording. The water maze tests began with visible platform training. For this purpose, the animal was first set, for 15 seconds, on a platform that was initially placed in the center of the pool and exposed 1 cm above the water surface. Then the animal was put into the water and allowed to swim/search for the platform and climb on it. The same procedure was repeated for 2 more trials, followed by the trials with the platform switched to each of the quadrants. After that, there were 5 consecutive days of learning, with each of the 4 trials per day beginning with the animal being placed into each of the 4 quadrants to initiate a 120-second trial. For each trial, the rat was placed in 1 of the 4 quadrants; if the rat found the platform in 2 minutes, it was permitted to rest there for 30 seconds before finishing the training. If the rat failed to find the platform in 2 minutes, it was led to the platform for a rest of 30 seconds. The latency of target finding and swimming distance (navigation test) was recorded.
On the day after completion of the navigation test, the platform was removed and the rat was permitted to explore the tank for 60 seconds The percentage of swimming distance in the target quadrant to total distance and the duration of the stay in the target quadrant were recorded as the indexes of the test (spatial probing).
Determination of BWC
Following cognitive tests, 12 rats in each time subgroup were further divided into 3 subgroups for histopathologic examination, BWC determination, and BBB-permeability assessment, respectively.
Four animals from each subgroup were subjected to BWC determination by using the dry-wet weight method.12 After decapitation, whole brain was weighed immediately to obtain the wet weight. Tissue was then dried in a desiccating oven at 100°C for 48 hours and reweighed to obtain the dry weight. BWC was calculated as the following: (wet weight − dry weight) / wet weight × 100%.
BBB-Permeability Assessment
Four animals from each subgroup were subjected to BBB-permeability assessment according to previously reported procedures.9 In brief, 2% EB dye (3 mL/kg) was injected into anesthetized rats through the femoral vein, and the brain was transcardially perfused 1 hour later. After decapitation, the brains were obtained and weighed and followed by incubation in formamide solution at 50°C for 72 hours. Optical attenuation of the EB formamide solution was determined by spectrophotometry at 635 nm, and BBB permeability was expressed as micrograms of EB per gram of brain tissue (microgram/gram).
IF Staining of GFAP
The death of 2 rats had reduced the sample number of planned histopathologic examinations for the subgroup of 40 Gy at 2 and 4 weeks' postirradiation, respectively. Animals were anesthetized and perfused; brains were removed and postfixed with 4% paraformaldehyde and processed for histopathology. Tissue sections (6-μm thick) were cut sequentially along the coronal profile. GFAP staining was performed as previously described,13,14 with the exception that a different primary antibody (1:100, Sigma-Aldrich, Hong Kong, China) was used in this study.
Quantitative image analysis was performed chiefly according to previously reported procedures.15 Serial coronal sections were examined for GFAP-positive astrocytes. For each animal, 3 parallel sections in the area of the hippocampus were evaluated for the number of stained cells and integral staining attenuation (the sum of all individual optical densities of each pixel in the area being measured). Images were acquired as digitized TIFF files and then were routed into a Windows PC for quantitative analyses by using Image-Pro Plus software, (http://www.mediacy.com/index.aspx?page=IPP) with the examiner blinded to sample identifiers. To maintain consistency across animals, we centered a rectangular box (0.72 × 0.58 mm) over the area of interest. The final results were shown as immunoreactivity per square millimeter.
Statistical Analysis
All the quantitative data were expressed as mean ± SD and were analyzed with the appropriate analysis of variance, followed by post hoc mean comparisons by using the Statistical Package for the Social Sciences, Version 12.0, software (SPSS, Chicago, Illinois). P < .05 was considered statistically significant.
Results
Five rats in the 40-Gy group and 2 in the 20-Gy group displayed mild local skin reactions, including depilation, hyperemia, and edema 6–15 days after the treatment. However, these conditions were not severe enough to prevent them from undergoing the behavioral tests and subsequent analysis. Two rats in the 40-Gy group had tooth deformities and oral mucosal ulceration and died at 2 and 4 weeks' postirradiation. All the rest of the rats were healthy. In addition, all rats showed normal daily activities, including feeding and drinking. No paralysis or convulsions were observed. Body weight slightly decreased in the first week after irradiation and returned to normal quickly. However, the changes did not reach statistical significance, and there was no difference between the groups.
Cognitive Deficits
The Morris water maze was used to assess the ability of place navigation and spatial probing. There was no difference in the swimming speeds for both parts. Place navigational function was impaired in both experimental groups. In the 20-Gy group, a transient impairment could be detected by a significantly increased latency (P < .05) and swimming distance (P < .05) compared with the sham controls only at 4 weeks' postirradiation. The cognitive performance returned to normal at 8 weeks' postirradiation. Compared with 20-Gy treatment, 40-Gy treatment induced more severe deficits, which became statistically significant (P < .01) by 4 weeks' postirradiation and lasted at least to 8 weeks (P < .05); then the rats returned normal at 12 weeks' postirradiation (Fig 1).
Effects of brain irradiation on the place navigation function of rats in a water maze. Rats exposed to different doses of fractionated irradiation treatments were subjected to the Morris water maze test. The latency of target finding (A) and swimming distance (B) was plotted and shown as indicated. One asterisk indicates P < .05; double asterisks, P < .01, compared with their corresponding sham.
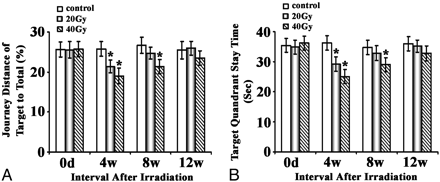
Results for the spatial probing test were essentially in line with those in the place navigation. The irradiation-induced effects reached statistical significance at 4 weeks in both the 20- and 40-Gy groups (P < .05); then, the rats recovered to normal at 8 weeks in the 20-Gy group and at 12 weeks in the 40-Gy group postirradiation (Fig 2).
Effects of brain irradiation on the space probe function of rats in a water maze. Rats exposed to different doses of fractionated irradiation treatments were subjected to the Morris water maze test. The journey distance of target to total (A) and target quadrant (B) staying time was plotted and shown as indicated. The asterisk indicates P < .05 compared with the corresponding sham.
Effect of Irradiation on BWC and BBB Permeability
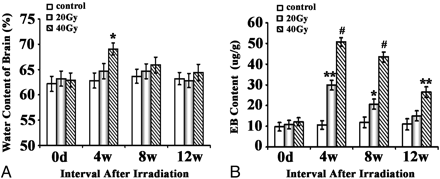
In the 20-Gy group, no significant increase of BWC could be observed during the whole experimental course. In the 40-Gy group, statistical significance (P < .05) was revealed at 4 weeks' postirradiation. This effect was transient and gradually returned to normal by 8 weeks.
Changes in the BBB permeability caused by the irradiation were more serious than the changes in the BWC. A statistically significant increase of BBB permeability was revealed 4 weeks' postirradiation (Fig 3) in both the 20- and 40-Gy experimental groups (P < .01 and P < .001, respectively). There was a trend toward gradual recovery. The subjects in the 20-Gy group returned to normal, but subjects remained significantly impaired in the 40-Gy group at 12 weeks' postirradiation. Furthermore, the deleterious effects of the 40-Gy treatment were significantly stronger than those of the 20-Gy treatment.
Effects of brain irradiation on BWC and BBB permeability. A, Water content of the brain is determined by the method of dry and wet weight. Water content in brain: (wet weight − dry weight) / wet weight × 100%. B, BBB permeability is expressed as micrograms of EB per milligram of brain tissue (microgram/gram). The asterisk indicates P < .05; double asterisks, P < .01; number sign, P < .001, compared with their corresponding sham.
Pathologic Changes
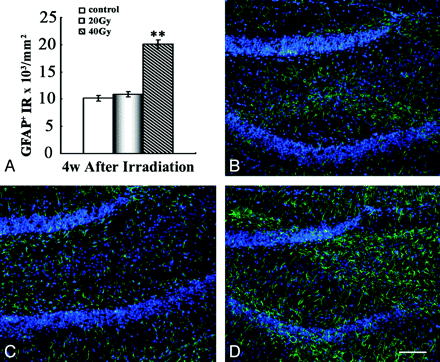
To better understand the potential changes of astrogliosis in animals after irradiation, we quantified the expression of GFAP, an astrocyte-specific intermediate filament protein, in the hippocampus. Our quantitative image analysis showed that in the 40-Gy group, significantly more GFAP+ astrocytes in the hippocampus could be observed at 4 weeks' postirradiation compared with the sham control group (P < .01). This change returned to normal by 8 weeks, while in the 20-Gy group, there was no change at any examined time points (Fig 4).
Increased GFAP+ astrocytes in the hippocampus after irradiation. A, Quantification of GFAP+ astrocytes in hippocampus. B−D, Representative IF results with anti-GFAP staining (green) in the hippocampus of the control group (B), the 20-Gy group (C), and the 40-Gy irradiated group (D) 4 weeks' postirradiation. Note a significant increase in GFAP+ fibers in the area of the hippocampus 4 weeks' postirradiation in the 40-Gy group. Scale bar is 100 μmol/L. Double asterisks indicate P < .01.
Discussion
Radiation therapy−associated neuroinjury of the central nervous system can be detected within hours after exposure to a dose higher than 15-Gy radiation, and fatality may occur within approximately 2 days.16 Acute RE, the radiation-induced brain injury, occurs in months after exposure to radiation and is a major health problem for patients. Cognitive dysfunction, with a dose-dependent impairment in working memory, is perhaps the most common sequela of acute RE.8,17,18 There is growing concern regarding the cognitive consequences of whole-brain irradiation for long-term cancer survivors.19
We have established an acute RE model in young rats undergoing single-dose whole-brain irradiation by using a linear accelerator before this study.9 However, most patients with cancer are treated with fractionated therapy currently, and there might be differences in the injury patterns between single-dose exposure and multiple fractioned exposures. To mimic the clinical protocol as closely as possible, young rats received a course of conventional whole-brain irradiation according to a schedule commonly used in the clinical setting in this study; and during the 3 months after irradiation, sequential behavioral and immunohistologic studies were observed in irradiation and sham groups.
Previous studies mainly focused on late-onset RE. Hodges et al19 reported radiation-induced deficits in T-maze forced-choices alternation and subsequent dose-dependent water maze deficits during a period of 44 weeks. They indicated that local cranial irradiation with low dose (20-Gy) of x-rays could produce cognitive deficits in adult rats without evidence of pathologic changes. Yoneoka et al20 found that fractionated irradiation at the 40-Gy level could cause memory deficits at 12 months after irradiation, but not at 6 or 9 months after irradiation in adult rats. Shi et al21 reported that radiation-induced spatial learning and memory deficits could be detected 12 months after irradiation in 12-month-old rats. However, the response to the radiation observed in young rats differed from that observed in old rats. Lamproglou et al22 studied the influence of age on learning and memory dysfunction induced by cranial radiation. They found that the radiation-induced (30-Gy) memory deficits could be detected at 1 month postirradiation in 4-month-old Wistar rats. Young rats showed an earlier decrease in learning and memory than older rats, and this deficit was followed by partial recovery.
In our study, we used young rats (4 weeks) for the tests, and our results suggest that progressive learning and memory dysfunction could be induced early after fractionated irradiation in young rats. This cognitive defect in the 20-Gy group was transient and could only be detected at 4 weeks' postirradiation, while in the 40-Gy group, this decline was more obvious with a longer duration compared with the 20-Gy group. The significant defects in cognitive function could be observed at 4 weeks and lasted at least until 8 weeks' postirradiation. In comparison with large single-dose irradiation reported previously,9 the cognitive impairment caused by the fractionated irradiation was less severe and started later and did not last as long.
Mechanisms of radiation encephalopathy remain to be elucidated. Shi et al21 indicated that an altered glutamate neurotransmission and/or excitatory neurotoxicity in the hippocampal CA1 region might be involved in the radiation-induced cognitive impairments. Other mechanisms, such as the death of vascular cells, changes in cytokines,23,24 reduction in regional glucose metabolism,25 and inhibition of the formation of new neurons in the hippocampus26 might also be involved. Considering that many proposed molecular mechanisms remain to be verified, the present acute model provides a means for further mechanistic study with a shorter experimental course than with a chronic disease model.
Change of BBB permeability has been proved to be the most sensitive and reliable index for detection of early radiation brain injury. Vasogenic edema following disruption of the BBB27 could be an early and readily recognizable pathophysiologic event occurring after irradiation. Nakata et al28 found that the destruction of the BBB was detectable as early as 1 day after irradiation with 20 or 40 Gy, reached its maximum after 3 days, and gradually decreased during the following few weeks. Yet to the best of our knowledge, no study of the destruction of the BBB and brain edema has been performed in the fractionated-radiation model. Our study revealed that the appearance of brain edema was transiently detected only at 4 weeks' postirradiation in the 40-Gy group. While the increased BBB permeability could be seen in both the 20- and 40-Gy groups and lasted much longer than brain edema, the deterioration for the higher dose group was much greater and lasted the entire course of the experiment. Therefore, the data here proved that the change in BBB permeability could be one of the most sensitive and reliable indices for the detection of brain injury in fractionated-radiation-induced acute RE. This is consistent with our previous observation in a single-dose-induced acute RE model.9
Some previous studies indicated that no abnormalities could be found at the light microscopic level in irradiated brains,20 and until now, less attention has been paid to the change of astrocytes. Our pathologic examination in the present study revealed an increase of GFAP+ astrocytes, with irregular distribution and morphology, in the area of the hippocampus after irradiation. This indicated transformation of astrocytes into reactive astrocytes.29 Astrocytes perform many functions, including biochemical support of endothelial cells, which form the BBB; provision of nutrients to nervous tissue; maintenance of extracellular ion balance; and the reparation and scarring process of the brain and spinal cord following traumatic injuries. Studies have suggested that reactive astrocytes occurring after an injury in the central nervous system may provide a permissive substratum to support axonal regeneration at the early stage of injury.30,31 Our results revealed that the reactive astrocyte gliosis was detected at 4 weeks' postirradiation in the 40-Gy irradiation group, which was in line with the cognitive dysfunction and increased BBB permeability, indicating that reactive astrocyte gliosis may be involved in the mechanism of irradiation brain injury.
Conclusions
The present study has demonstrated that acute brain injury can be produced in young rats after receiving cranial fractionated radiation, which is accompanied by brain edema and disruption of the BBB. Reactive astrocyte gliosis was thought to play an important role in this pathologic process. In addition, higher dosage (40 Gy) induced more serious change, which lasted longer compared with that induced by a lower dosage (20 Gy). The present model, especially in subjects receiving 40-Gy irradiation, will be useful in elucidating the pathogenesis of acute radiation-induced cognitive dysfunction and can be used for the relevant mechanistic and therapeutic studies.
Footnotes
Haihong Zhou and Zhonglin Liu contributed equally to this work.
-
This study was supported by a grant to Jun Liu from the National Natural Science Foundation of China (No. 30870750)
Indicates open access to non-subscribers at www.ajnr.org
References
- Received December 20, 2010.
- Accepted after revision March 3, 2011.
- © 2011 by American Journal of Neuroradiology