Abstract
BACKGROUND AND PURPOSE: FDs are the latest, most promising tool to treat giant and complex aneurysms. Currently available experimental aneurysm models do not reproduce the potential clinical difficulties of treating these lesions with FDs.
MATERIALS AND METHODS: Six large or giant canine fusiform aneurysms were created on the distal carotid arteries of 4 animals. Four of the aneurysms had multiple arterial branches originating from the aneurysm; 2 other aneurysms had all branches clipped at the time of aneurysm construction. Aneurysms were treated with multiple telescoping prototype flow-diverting stents (total of 15 FDs). Angiography was carried out before and immediately after implantation, at 2 weeks, and immediately before sacrifice at 12 weeks. Macroscopic photography of specimens was performed, followed by biopsies of selected regions of the tissue formed on the surface of FDs.
RESULTS: Technical or device-related difficulties occurred in 2 of 6 aneurysm treatments. Fusiform aneurysms with branches intact remained widely patent (mean angiographic score, 3), whereas aneurysms with clipped branches had only small residua (mean angiographic score, 1) at 12 weeks. The presence of very small defects in neointima formation on the surface of FDs, or leaks, was sufficient for residual filling of the aneurysms, which served as reservoirs to feed branches.
CONCLUSIONS: Experimental canine fusiform carotid aneurysms may reproduce many of the difficulties associated with the treatment of giant aneurysms and could be appropriate for preclinical testing of FD stents.
ABBREVIATIONS
- ASA
- acetylsalicylic acid
- FD
- flow diverter/diversion
- H&E
- hematoxylin-eosin
Endovascular FDs are the latest and most promising tools to treat intracranial aneurysms. Initial use of these tools in otherwise untreatable aneurysms has led to some spectacular successes,1,2 bolstering enthusiasm in the endovascular community.1⇓–4 However, as complications related to FD use accrue,5,6 there is an increasing need for preclinical experimental evidence to guide the use of these new devices and to test future FDs before clinical introduction. Most clinically encountered complications, other than those due to access or technical difficulties with deployment, are a result of the increased thrombogenicity of these devices or are related to occlusion of jailed branches or perforators, although hemorrhages postflow diversion have been identified and are increasingly reported.5,6 Failure of these devices to lead to aneurysm occlusion also has been documented,7 though the significance of angiographic failure to achieve complete occlusion using this new technology remains unclear.
The impact of FDs on aneurysms and branches has so far been the object of studies using conventional experimental aneurysms,10 but these in vivo models, developed to test embolic material such as coils,11 are not challenging enough to mimic clinical conditions in which FDs are currently used. Types of aneurysms most frequently treated with FDs12,13 are large, fusiform lesions, often untreatable by other means.
We described a lingual artery bifurcation aneurysm model previously.12,13 The presence of multiple external carotid branches allows the construction of a larger, fusiform aneurysm on a segment bearing multiple arterial branches. An aneurysm model that reproduces some of the potential difficulties encountered with FD use in these difficult aneurysms can thus be created in experimental in vivo conditions. The first use of the new model was to assess whether FDs could simultaneously occlude fusiform aneurysms but preserve the arterial branches and to address the potential relationship between aneurysmal and arterial branch flows, by ligation of branches originating from the arterial segment on 1 side in animals with bilateral aneurysms.
We report for the first time how flow diverters can fail to occlude in vivo experimental giant aneurysms, despite the presence of multiple layers of FDs, and the role of arterial branch flow in maintaining aneurysmal patency. We also report some technical difficulties that can arise from FD use in treating complex aneurysms.
Materials and Methods
Surgical Aneurysm Creation
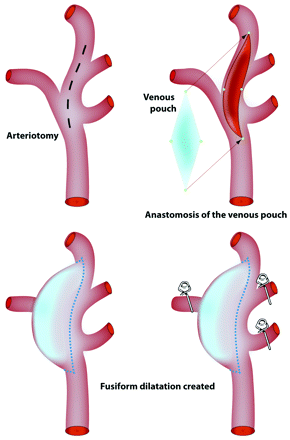
Protocols for animal experimentation were approved by the Institutional Animal Care Committee in accordance with guidelines of the Canadian Council on Animal Care. All procedures were performed in 7–10-kg beagles under general anesthesia. In total, 6 fusiform lingual aneurysms were constructed in 4 animals. Through a midline vertical cervical incision, the left external jugular vein was harvested and stored in heparinized saline until time for aneurysm creation. In the first 2 animals, 1 distal carotid artery was exposed in the region of the lingual artery, and temporary clips were applied to all efferent branches (lingual, pharyngeal, occipital, facial, and maxillary). A 2–2.5-cm linear arteriotomy was created, and an ellipse of the harvested vein was anastomosed with continuous 7.0 Prolene suture (Ethicon, Cincinnati, Ohio), as for patch repair after carotid endarterectomy, to create a fusiform dilation of the vessel. Temporary clips were removed and bleeding points repaired with sutures. After successful unilateral aneurysm creation in the first 2 animals, the subsequent 2 dogs had bilateral fusiform aneurysms created. However, animals with bilateral aneurysms had the right-sided aneurysm created as described above, whereas all efferent branches originating from the left-sided aneurysm were occluded with surgical clips. Incisions were closed in multiple layers over Penrose drains. (Fig 1)
Surgical construction of the model. Schematic depicting surgical construction of lingual fusiform model with or without branches, with venous patch graft remodeling to form large or giant aneurysms.
FDs
FDs were gifts from Microvention (Aliso Viejo, California). These devices are flexible, microcatheter-delivered, self-expanding cylindrical constructs made of an inner sleeve of low-porosity/high pore-density mesh attached inside a conventional outer high porosity stent. FDs were of variable lengths (measured lengths of 26–52 mm in 3.0-mm glass tubes) but identical construction (wire 0.001-inch outer diameter, in vitro porosity of 71%, and pore density of 10.4 pores/mm2, values measured in 3.5-mm glass tubes; data not shown).
Endovascular Treatment
Endovascular treatment was performed 4 weeks or more after surgical aneurysm construction. All animals received 325 mg of ASA and 75 mg of clopidogrel for 4 days before stent implantation. Percutaneous transfemoral angiography was performed, and the intended recipient artery diameter and dimensions of each fusiform lingual aneurysm were measured. The number of branches originating from each aneurysm also was recorded. Microcatheters (inner lumen 0.027) were coaxially inserted into 6F guiding catheters positioned in the common carotid artery. FDs were deployed from the distal efferent vessel (the maxillary artery) extending back into the proximal common carotid artery. Multiple FDs were used to bridge the aneurysms, with a landing zone of at least 12–15 mm on either side of the aneurysm. Clopidogrel therapy was continued for 10 days poststent implantation, whereas 325 mg/day ASA was continued until sacrifice.
Angiography
Transfemoral angiography was performed in all animals immediately before and post–stent deployment, at 2 weeks, and immediately before sacrifice at 12 weeks. To prevent femoral hematomas on antiplatelet agents, all punctured femoral arteries were surgically exposed through a small linear incision and ligated.
Angiographic results were scored on a 4-point scale, in which 3 represents no change in aneurysm flow, 2 represents partial occlusion, 1 represents nearly complete occlusion, and 0 represents complete aneurysm occlusion (modified from Kamran et al14).
Macroscopic Photography and Pathology
Euthanasia was performed by barbiturate overdose and followed by en bloc resection of the aneurysm and parent artery. After fixation in 10% formalin, the fusiform lingual aneurysm construct was opened longitudinally and photographed by using a computerized imaging system (Vision PE Clemex Technologies, Montreal, Quebec, Canada), paying attention to the presence of organized or unorganized intra-aneurysmal thrombus, location and quality of neointima formation on the stent surfaces, and the fate of stented branches originating from the aneurysmal segment. Additional dissections and photographs were performed as needed. To circumvent artifacts due to the several layers of metallic foreign material, selected samples of tissue coverage over areas of interest were biopsied under the operating microscope. Biopsy specimens were stained with H&E and Movat pentachrome, followed by immunohistochemistry with smooth muscle cell α-actin and endothelial factor VIII.
Results
All surgical and endovascular procedures were well tolerated. Aneurysm characteristics and results of endovascular treatments are summarized in the Table. All aneurysms and branches were found to be patent before stent placement, other than the intentionally clipped branches, which filled retrogradely through collateral flow. While the first FD was being implanted, despite having ensured at least 15-mm landing zones, the distal end of the FD retracted and prolapsed back into the aneurysm. The stent was recatheterized and a second stent was used to bridge the distance between the distal maxillary artery and the more proximal stent. In the second animal, a single 52-mm stent was sufficient to bridge the entire aneurysm, with ample landing zones beyond the aneurysmal segment. Nonetheless, at angiography 2 weeks later, both of these stent constructs were seen to have retracted and prolapsed back into the aneurysm. The timing of this unexpected complication is difficult to determine, because both construct failures were asymptomatic.
Angiographic and pathologic outcomes after flow diversion in experimental fusiform aneurysms
Subsequent aneurysms were thus planned to be treated with multiple telescoping stents, to cover much longer segments (3–5 cm) of the parent vessel on both sides of the aneurysm, to ensure complete permanent reconstruction from the distal maxillary to the proximal carotid artery. The next 2 animals (with bilateral aneurysms), had 4 FD constructs implanted, each consisting of 2–4 telescoping devices. All of these latter constructs were found to be stable at 2 and 12 weeks.
Angiographic Results
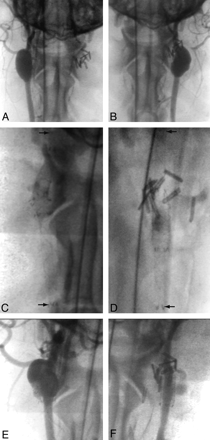
At 2 and 12 weeks, all stented parent vessels were found patent. Minimal irregularities without significant luminal narrowing were found in some stented arterial segments, particularly when multiple layers of FD stents were present. All fusiform aneurysms with arterial branches originating from the aneurysms remained widely patent despite FD treatment (angiographic score 3). However, the 2 aneurysms whose branches were occluded at time of surgical construction had progressive occlusion of the aneurysm and only minor residual aneurysm filling at 12 weeks (angiographic score, 1; Fig 2).
Angiographic results of treatments. Angiographic demonstration of giant fusiform aneurysm with (A) or without (B) arterial branches, 6 weeks after construction, immediately before treatment. Both have been treated with multiple FD constructs to span the aneurysmal dilation (arrows in C and D). Stent placement in the aneurysm with arterial branches led to treatment failure (E), whereas, in the aneurysm with surgically occluded branches led to almost complete aneurysm thrombosis after FD (F).
Pathology
All parent vessels as well as branches were found patent at pathology. FD stents were well apposed to the parent arteries and were covered with a 50–300-μm mature neointimal layer, with no significant luminal narrowing. Stent extremities, composed of nitinol wires covered with platinum markers, were sometimes the site of small, irregular less mature neointimal nodules. There was organized thrombus occluding the distal end of the 2-stents construct that had retracted inside the aneurysmal pouch in animal 1, but all other FD stents were fully patent.
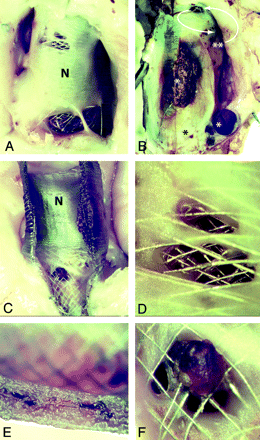
There was no thrombus in aneurysms 1–4 (with branches), but aneurysms 5 and 6 were 80% occluded, with thrombotic occlusion of aneurysmal loculations of different ages and at varying stages of organization (Fig 3).
Residual aneurysm, neointima formation, and leaks. Note patent, open aneurysms (A; corresponding to Fig 2E), when the aneurysm serves as a reservoir for feeding branches. When branches are absent (B) the aneurysm is almost completely occluded, with pouches containing thrombus in various stages or organization (*). A small crescentic remnant, fed by a small leak (curved arrows) is present (**; aneurysm corresponding to Fig 2F). The FDs are covered with neointima both inside and outside (C), and leaks penetrate both neointimal layers covering the stents (D and E). There is good neointimal coverage of the stent struts apposed to the parent artery (E). Note patent arterial branch ostium but partially covered with neointimal tissue and organizing clot (F).
Residual aneurysms, large or small, had a thickened wall. The most striking finding was that FD stents were covered with thick, mature neointimal tissue both inside and outside the stent construct in all cases of residual aneurysms. Closer inspection revealed the presence of minute holes, or leaks in the neointimal tissue layers, 200–900 μm, that often traversed multiple layers of scaffolding metal mesh. The amount of blood flow that “leaked” through these holes, whose small size could not have been predicted with angiography, was apparently sufficient to maintain patency of the aneurysms, as well as of branches (when they were not clipped).
In some cases, the neointimal tissue at the site of the hole was of an irregular contour, and composed of tissue of a reddish brown color, compared with the more uniform, whitish tissue found elsewhere on the FD stent (Fig 3).
Discussion
Model
This initial work by using a new animal model has shown that large fusiform aneurysms can be constructed in canines to test FDs in a context that is more closely related to their clinical use. The model can reproduce various technical difficulties that are intrinsic to this type of treatment, such as immediate or delayed stent prolapse into the aneurysm.
Unlike smaller experimental aneurysm models, this fusiform model reproduces the difficult-to-treat clinical situation where a long segment of the FD is not in contact with the parent artery, allowing the self-expanding FD to continue to expand. Although the model differs from the human clinical context in multiple ways, it meets a fundamental need of all useful models: to recreate the clinical problem we hope to address in the relatively controlled environment of the laboratory.15
In addition to rendering difficult the measurement of the appropriate length of stent required to bridge the aneurysm, the expansion of the FD into the potential space of the aneurysm places an additional force on the stent, leading to a tendency for the stent to “squirt” back into the aneurysm if the landing zone on either side of the dilated segment is insufficient. The final length of self-expanding woven constructs will depend on the maximal diameters the device will be allowed to reach. Hence, it may become difficult to estimate how long the stent construct should be before actual deployment. Although FD prolapse back into the aneurysm may occur immediately upon deployment, possibly as a result of the device being stretched excessively to bridge the aneurysmal segment of the artery, retraction of FDs also can occur in a delayed manner, as we saw in our first 2 cases.
The prototype FDs used in this study are manufactured to capture, in a single device, the FD-in-stent technique sometimes described for the treatment of difficult human fusiform aneurysms.7,16 The idea is to prevent excessive expansion of the weak FD stent with a stronger, more conventional stent. Despite using this strategy, multiple telescoping stents were required (up to 4 in 1 case) to prevent intra-aneurysmal prolapse.
The use of FD stents in this model failed to occlude aneurysms. Excluding the first 2 cases, where failure can be attributed to technical or device-related problems, the results for the 4 remaining aneurysms can be clearly dichotomized according to whether or not there were branches originating from the aneurysms.
Although the role of continued blood flow in maintaining arterial patency of branches jailed by FDs has been suggested by computational fluid dynamics studies,17 the findings of this initial work remain striking: very small open pores, or leaks, in the thick neointima covering the FD were sufficient to preserve flow both in residual aneurysms and in arterial branches as well. This phenomenon was observed despite multiple (up to 4) layers of dense mesh coverage. The persistence of these small leaks along with intact or enlarging aneurysms at 3 months is preclinical evidence that this strategy may not always be effective when it is the aneurysm itself that feeds the collateral branches essential to cerebral blood flow.
Limitations
The small number of aneurysms and animals used for this initial study cannot be used to support universal generalizations or meaningful statistics. Results however are sufficiently compelling to demonstrate that failures can occur, and mechanisms can be explored by using this model. End points at 3 months may be too early to judge the final efficacy the endovascular strategy could have had, had the animals been observed for a longer period. This may be particularly true for those aneurysms without branches, which may have completely occluded with longer observation time. In aneurysms with branches, success could perhaps have been assured by using a larger number of telescoping, overlapping FD stents, but with each additional layer of metal, we feared risks of parent artery thrombotic complications. Given the costs of the multiple flow diverters implanted, as well as the risk of parent vessel thrombosis, we felt this limited study sufficed to show that FDs can fail to occlude fusiform aneurysms that include the origins of arterial branches, through leaks that are well- formed holes in mature neointima. Hence, we speculate that these leaks are likely to be stable and remain open for any future length of time.
The use of multiple overlapping devices to treat these lesions certainly introduced some differences in metal coverage of the aneurysms, and may have led to some differences in flow within aneurysms with branches compared with those without. The impact of this difference in metal surface area, though theoretically possible, is unlikely to account for the large differences in angiographic filling between those aneurysms with branches as compared with those without branches.
The antiplatelet regimen we have used is inspired from human clinical experience, but of course dosage and timeframes are somewhat arbitrary. Preliminary experience with these FDs in other models has shown that double antiplatelet therapy is essential to prevent parent artery thrombosis (data not shown). The presence of pre-existing thrombus within an aneurysm, as often occurs with human aneurysms, also may alter the response of the aneurysm to treatment.
Measuring angiographic outcomes after treatment with flow diverters remains a difficult problem. Angiographic results are surrogate outcomes and one could argue about the definition of success or failure after FD therapy. Noninvasive imaging modalities such as plain films or CT scan may have been helpful to better determine the timing of device prolapse.
We considered that the presence of any circulating intraaneurysmal blood flow was a failure of treatment, but this may be too stringent a criterion. Perhaps for clinical applications, the reduction of mass effect or even hemorrhagic risks might occur in giant aneurysms treated with FDs without requiring complete occlusion of the aneurysm. This possibility remains, at least for the time being, a speculation difficult to prove.
Conclusions
Giant fusiform aneurysms can be constructed in canines and could be helpful in the preclinical assessment of FDs. FDs can fail to completely occlude aneurysms through gaps in the neointimal coverage that forms over the surface of the device. The resulting leak through the FD construct can serve to feed arterial branches that originate from the aneurysmal segment of the parent artery.
Footnotes
-
Disclosures: Tim E. Darsaut: Research Support (including provision of equipment or materials): Society for Interventional Radiology (SIR), Details: Pilot grant: 1 year; US $25 000; Jean Raymond: Research Support (including provision of equipment or materials): Microvention, Details: FDs were gifts from Microvention (Aliso Viejo, California).
References
- Received January 31, 2011.
- Accepted after revision March 21, 2011.
- © 2011 by American Journal of Neuroradiology