Abstract
BACKGROUND AND PURPOSE: Open MR imaging scanners are designed for imaging of specific patient groups that cannot be routinely scanned with conventional MR imaging scanners (eg, patients with obesity and claustrophobia). This study aims to determine whether BOLD sensitivity on an open 1T scanner is adequate for fMRI for diagnostic and research purposes by directly comparing fMRI results with a standard 3T MR imaging scanner. The optimal TE was also determined.
MATERIALS AND METHODS: Twelve healthy adults were scanned by using both an open 1T scanner and a standard 3T scanner. Gradient-echo echo-planar images were acquired for all subjects while performing motor and affective paradigms, each at 5 different TEs per scanner (range, 40–80 ms at open 1T; 20–40 ms at 3T). To compare BOLD sensitivity between scanners and TEs, we determined maximum statistical t scores per TE for all relevant brain areas (motor cortex, visual cortex, amygdala, and OFC) for individual subjects and group analyses. Additionally, T2* values were determined per scanner for the relevant brain areas.
RESULTS: Maximum t scores were significantly lower in the relevant brain areas on the open 1T compared with the 3T for single subjects but not for group analyses. The optimal TE for fMRI on an open 1T MR imaging system was found to be approximately 70 ms.
CONCLUSIONS: Although for single-subject studies as used in diagnostics, 3T was found to be superior, fMRI on an open 1T MR imaging scanner is suitable for research designed to analyze data at a group level.
Abbreviations
- AAL
- automated anatomic labeling
- BOLD
- blood oxygen level–dependent
- EPI
- echo-planar imaging
- FA
- flip angle
- fMRI
- functional MR imaging
- MNI
- Montreal Neurological Institute
- Nd
- nondetectable
- OFC
- orbitofrontal cortex
- TE
- echo time
- TR
- repetition time
Open MR imaging scanners are designed to scan specific patient groups that cannot be routinely scanned with conventional MR imaging scanners (eg, patients with obesity and claustrophobia and young children) and to facilitate performing interventions while scanning (eg, lumbosacral injections).1 In particular, the rising number of patients with morbid obesity increases the need for adequately sized equipment for this patient group.2,3 This need can be met by the availability of vertical-field open MR imaging scanners with vertical instead of cylindrical bores. However, advanced applications, such as fMRI, are not yet well-explored on open MR imaging scanners. fMRI is used to investigate neuronal activity by measuring BOLD contrast in the brain and can be conducted on conventional MR imaging scanners in both clinical and research settings. Successful application of fMRI on an open MR imaging scanner could make this technique available for the above-mentioned patient groups.
Most fMRI studies have been conducted on conventional MR imaging systems with a magnetic field strength of ≥1.5T, whereas the current standard for fMRI is at 3T. In contrast, the maximum available magnetic field strength of open MR imaging systems is currently only 1T. Magnetic field strength affects BOLD sensitivity and has a positive linear relationship with the signal intensity–to-noise ratio.4,5 Therefore, it is questionable whether an open MR imaging system with a lower magnetic field strength can be used for fMRI. To date, only a small number of studies have been published using fMRI with standard 1T scanners,6–9 testing motor,6,8,9 visual,7 language,6 and executive9 functions. Whereas these studies indicated that fMRI is feasible for these functions at a magnetic field strength of 1T, to our knowledge, fMRI has not been tested on an open MR imaging scanner.
Apart from the magnetic field strength, various other scanning parameters may also affect the BOLD contrast. An important parameter is TE. At the optimal TE, contrast in T2* relaxation is maximal between brain regions with low and high levels of deoxyhemoglobin concentration, assumed to reflect differences in regional neural activity.10 Theoretically, the optimal TE coincides with the local T2* but also depends on the magnetic field strength of the scanner.
The aim of this study was 2-fold. The first aim was to investigate whether BOLD sensitivity on an open MR imaging scanner with a field strength of 1T is suitable for fMRI for diagnostic and/or research purposes. The second aim was to identify the optimal TE for fMRI on the open 1T scanner. To this end, both motor and affective paradigms were performed by healthy subjects while being scanned at different TEs in an open 1T MR imaging scanner, the results of which were compared with those from a standard 3T MR imaging scanner, also acquired at different TEs.
Materials and Methods
Subjects
Twelve normal-weight healthy adults without claustrophobia (5 men, 7 women; age, 26.7 ± 2.4 years; range, 23–32 years; 10 right-handed, 1 left-handed, 1 ambidextrous) were included in the study. None of the subjects had a history of head trauma, seizures, or brain pathology; a current psychiatric or neurologic illness; or current use of psychotropic medication. All subjects gave written informed consent for the study, which was approved by the local medical ethics committee.
Experimental Design
All subjects underwent 2 scanning sessions on an open 1T whole-body scanner (Panorama; Philips Best, the Netherlands) and 1 scanning session on a 3T whole-body scanner (Intera; Philips Healthcare) on different days. In both scanners, the subjects performed a motor paradigm 5 times and an affective paradigm 5 times, both scanned at 5 different TEs in random order. The motor paradigm was a finger-tapping task consisting of 5 rest blocks alternating with 5 blocks of right-handed finger-tapping, cued by a visual stimulus. During each block, 10 functional MR imaging scans were acquired, resulting in 100 scans per task. The affective paradigm consisted of 5 blocks of neutral pictures, 5 blocks of positive valence pictures, and 5 blocks of pictures of negative valence, presented in pseudorandom order. Positive and negative valence pictures were intended to induce positive and negative emotions, respectively, by displaying, for example, a happy child or a disgustingly dirty toilet. Pictures were selected from the International Affective Picture System data base (csea.phhp.ufl.edu/media.html). Five different versions of the affective task were used so that subjects viewed each picture only once, to control for habituation effects. Each picture was shown for 3500 ms. Each block lasted 10 scans, resulting in 150 scans per session.
Imaging Parameters
During each paradigm, gradient echo-planar images were acquired with optimal protocol settings for both scanners (Fig 1). Scanning parameters for the open 1T scanner were the following: TR, 3200 ms; FA, 90°; matrix, 64 × 64; voxel size, 3.4 × 3.4 × 4.5 mm; 27 sections; no parallel imaging; gap, 0.45 mm; ascending scan order. On the open 1T scanner, a 4-channel head coil was used. Scanning parameters for the conventional 3T scanner were based on our standard fMRI protocol: TR, 2600 ms; FA, 90°; matrix, 96 × 96; voxel size, 2.3 × 2.3 × 3.0 mm; number of sections, 40; parallel imaging (sensitivity encoding) factor, 2.5; gap, 0.3 mm; ascending scan order. On the 3T scanner, an 8-channel head coil was used. To determine the optimal TE, a range of 5 different TEs was chosen per scanner (open 1T: 40, 50, 60, 70, 80 ms; 3T: 20, 25, 30, 35, 40 ms). Additionally, T2* maps were acquired on both scanners as an extra tool to determine the optimal TE per scanner by using a multiecho 2D gradient-echo sequence (TR, 110 ms; 20 echoes; echo spacing, 4 ms starting at 1.6 ms; FA, 30°; other scanning parameters were identical to the EPI protocols). High-resolution T1-weighted 3D images (TR/TE, 9.8/3.3 ms; FA, 8°; matrix, 256 × 256; voxel size, 1.2 × 1.2 × 1.2 mm; number of sections, 120) were acquired on the conventional 3T MR imaging scanner for anatomic reference.
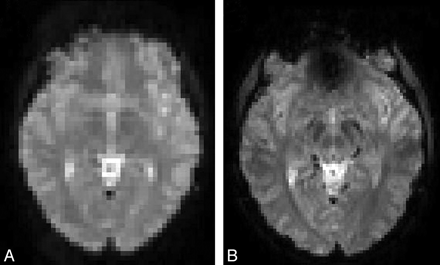
Examples of EPI sections of the same subject for the open 1T MR imaging scanner (A) and the 3T MR imaging scanner (B).
Statistical Analysis
Functional scans were analyzed by using SPM5 (Wellcome Department of Imaging Neuroscience, London, United Kingdom). All scans underwent slice-timing correction, motion correction, coregistration to the anatomic reference scan, and normalization to the standard MNI 152 brain, including resampling to 3.0-mm isotropic voxels and spatial smoothing (Gaussian kernel, full width at half maximum = 8 mm). One scanning session was excluded from analysis because of motion exceeding 2 mm (affective task scan on an open 1T scanner; TE, 40 ms). Next, a statistical t map was created per individual for each scanning session (ie, per TE for both motor and affective paradigms). Maximum statistical t scores were determined in preselected brain areas—that is, left motor cortex (AAL: left precentral gyrus) for the motor paradigm, and visual cortex (AAL: left and right calcarine sulcus, left and right lingual gyrus, left and right cuneus, left and right inferior and medial occipital cortex), amygdala (AAL: left and right amygdala), and OFC (AAL: left and right superior, medial, and inferior OFC) for the affective paradigm. These areas are known to show BOLD signal-intensity changes by the paradigms used.
For the single-subject analyses, the mean maximum statistical t scores for 12 subjects were calculated for each area of interest for each TE. In addition, group analyses of the 12 subjects were performed for each TE for both paradigms by a mixed-effect 1-sample t test analysis. On the statistical t maps of the group analyses, the maximum statistical t scores were again determined in the preselected brain areas. For both single-subject and group analyses, activity-correlated signal-intensity changes were considered undetectable when none of the voxels in the preselected brain areas reached a t value > 1.65 (P > .05, uncorrected). Maximum t scores per brain area were used to compare BOLD sensitivity among different TEs and between the 2 scanners, both at single-subject and group levels. For comparison of the results of the single-subject analyses between the 2 scanners, a t test was used to compare the highest mean maximum t scores per brain area. For comparison of the results of the group analyses between the 2 scanners, the group results of the TE with the highest t scores were compared with a paired-samples t test in SPM5 for each brain area.
The T2* maps were analyzed in the Functional MR Imaging of the Brain Software Library (www.fmrib.ox.ac.uk/fsl). On the basis of the signal-intensity decay in the multiecho readout, we calculated the T2* value per voxel. The maps were coregistered to the standard MNI 152 brain. Next, an average T2* map of the 12 scans for each scanner was made, to allow comparison of T2* values between various field strengths. The T2* values for the voxels with the same MNI coordinates as the voxels in which the highest t scores were found for the relevant brain areas were determined on the basis of the average T2* maps for both scanners.
Results
Task-correlated signal-intensity changes were detectable in the left motor cortex and visual cortex in all individual subjects for all TEs on both scanners. On the open 1T scanner, no task-correlated signal-intensity changes were detectable in 14 of 59 sessions in the right amygdala, 9 of 59 sessions in the left amygdala, 4 of 59 sessions in the right OFC, and 8 of 59 sessions in the left OFC. On the 3T scanner, there were no detectable task-correlated signal-intensity changes in 6 of 60 sessions in the right amygdala, 11 of 60 sessions in the left amygdala, and 3 of 60 sessions in the right OFC. In the left OFC, signal-intensity changes were detectable in all scans on the 3T scanner. Sessions in which the task-correlated signal-intensity changes were nondetectable were evenly distributed over the subjects.
When comparing the results on the open 1T scanner with those on the 3T scanner, we found the maximum t scores per TE for the single-subject analyses to be lower on the open 1T than on the 3T scanner (On-line Table) for all brain areas (left motor cortex, P = .002; right visual cortex, P = .006; left visual cortex, P = .002; right OFC, P = .018; left OFC, P = .001) except for the amygdala (right, P = .294; left, P = .351). In the group analyses, the t scores were generally lower on the open 1T (Fig 2), but this difference was only significant for the right OFC (P < .001).
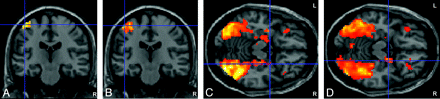
A and B, Examples of left motor cortex activation (crosshairs) in a group analysis for the open 1T (TE = 70 ms, A) and the 3T scanner (TE = 40 ms, B), (P < .05, family-wise error−corrected). On both scanners, the activation is well-detected. Notice the larger size of the activation area on the 3T scanner (B). C and D, Examples of right amygdala activation (crosshairs) in a group analysis for the open 1T (TE = 70 ms, C) and 3T scanners (TE = 20 ms, D), (P < .001, uncorrected). Right amygdala activation is well-detected on both scanners. Similarly, extensions of the visual cortex activation and OFC activations can be seen for both scanners. Notice the larger activation areas on the 3T scanner (D). Images are in neurologic orientation.
The maximum t scores per brain area in the individual and group analyses were found at different TEs on both scanners. On the open 1T scanner, the highest t scores in the left motor cortex were found at TEs of 70 and 80 ms; in the visual cortex and in the amygdala, at 50, 70, and 80 ms; and in the OFC, at 40, 60, and 70 ms (On-line Table). On the 3T scanner, a TE of 40 ms resulted in the highest t scores in the left motor cortex, whereas for the visual cortex, the highest t scores were found at 20, 25, and 40 ms. For the amygdala, the highest t scores were found at 20, 25, 30, and 35 ms and for the OFC at 20 and 30 ms (On-line Table).
The average T2* values per brain region are shown in the Table. For the open 1T scanner, these varied from 68 ms in the right visual cortex to 85 ms in the left motor cortex and for the 3T scanner, from 35 ms in the left amygdala to 55 ms in the left motor cortex.
Average T2* values per brain region (in milliseconds)
Discussion
On the open 1T MR imaging scanner, task-related signal-intensity changes were detectable in all brain areas of interest with the group analyses. Only in the left amygdala, the activity was not detectable at 2 TEs, though at the other 3 TEs, it was detectable with t scores up to 5.7. For the individual analyses, task-related signal-intensity changes in the amygdala and OFC were detectable in fewer subjects on the open 1T scanner than on the 3T MR imaging scanner. Also, t scores per brain area were significantly lower on the open 1T scanner for all areas, except the amygdala. In addition, t scores in the amygdala and OFC were low to moderate (t <3.0), below standard thresholds for multiple comparisons even within a region-of-interest approach. These findings, therefore, indicate that the open 1T scanner lacks adequate sensitivity to detect task-related signal-intensity changes in the amygdala and OFC in individual subjects.
In contrast to these single-subject results, group analyses failed to show significant differences between data acquired on both scanners, with the exception of right OFC activity during presentation of emotional pictures, though the t scores per brain area were generally lower on the open 1T scanner than on the 3T MR imaging. This finding indicates that at a group level, loss of BOLD sensitivity on the open 1T scanner is only modest compared with a state-of-the-art 3T scanner. A likely explanation is that for single-subject analyses within-subject variance is a key factor, whereas for random effects group analyses, between-subject variance is essential. In the present study, between-subject variance was similar for open 1T and 3T systems, analogous to results from studies comparing fMRI on 1.5T and 3T MR imaging scanners.11–13
In the present study, we chose to compare 1T versus 3T results on the basis of optimal settings for each platform. Results for different TEs showed that for the open 1T scanner, a TE of 70 ms resulted in overall highest t scores across brain areas for the group analyses. In the single-subject analyses, the highest t scores were found at 80 ms for brain areas that are not prone to susceptibility artifacts (left motor cortex and visual cortex) and at lower TEs (50–70 ms) in areas where susceptibility artifacts may affect the signal intensity (amygdala, OFC). From these results, we may conclude that overall, a TE of 70 ms is presumably optimal for detecting BOLD contrast for an open 1T MR imaging scanner. However, when focusing on neural activation in the motor or visual cortex, especially in individual studies, one may consider choosing a TE higher than 70 ms. In contrast, when regions of interest include the amygdala or OFC, one should choose a TE lower than 70 ms. This suggestion is in line with previous research indicating that regions that are affected by susceptibility-induced BOLD sensitivity losses should be scanned with a reduced TE, due to faster signal-intensity decay in those regions.14
In contrast to these findings on an open 1T system, in the present study, establishing an optimal TE for the 3T scanner proved to be less straightforward. Group analyses showed that the highest t scores in the amygdala and OFC were obtained at or below a TE of 30 ms, whereas for the left motor cortex the highest t scores were found at 40 ms, and in the visual cortex, at both 20 and 40 ms. Single-subject analyses revealed a similar pattern with an optimal TE for the motor cortex at 40 ms but with TEs below 30 ms for the other brain areas. Therefore, a TE below 30 ms is generally advisable when performing fMRI on a 3T scanner, except when focusing on dorsolateral cortical areas such as the motor cortex.
Contrary to our expectations, for the 3T scanner, the TE curves did not show a unimodal pattern (ie, a single optimal TE for each brain region). Fera et al11 have reported previously that there is likely to be a broad range of optimal TEs when scanning subjects who perform motor tasks at a TE range of 30–200 ms on 1.5T and 3T scanners. In the present study, the wider range in TEs that we chose for the open 1T scanner (40–80 ms) may explain why we were able to determine optimal TEs for this scanner but not for the 3T scanner. In addition, we observed large intraindividual variability between sessions, which appeared to be independent of TEs. McGonigle et al15 have shown earlier that within-subject between-session differences are an inherent part of the fMRI technique and should be taken into account when evaluating a single session of a single subject.16
The T2* values for the open 1T MR imaging scanner were observed to range from 68 to 85 ms, in agreement with our findings that a TE around 70 ms is optimal for the current fMRI protocol. Most surprising, the lowest T2* values on the open 1T scanner were found for the visual cortex, which is not particularly prone to susceptibility-induced signal-intensity decay. Possibly, this finding may reflect the fact that at a lower field strength susceptibility-induced signal-intensity drop-out is reduced. For the 3T, the broad TE optimum that we observed only partly overlaps with the range in T2* values that we found; the fastest signal-intensity decay was in the amygdala as expected due to susceptibility artifacts in this region.
This study tested the BOLD sensitivity on an open 1T MR imaging system for motor and affective paradigms. These paradigms were chosen because they are well-validated and known to induce robust BOLD signal-intensity changes in specific brain regions, including areas that are prone to susceptibility artifacts. However, it remains to be established whether similar results can be obtained for other paradigms. Another potential limitation of this study is that the scans on open 1T MR imaging were acquired in 2 subsessions compared with 1 session on the 3T MR imaging. This was done because the overall scanning protocol on the open 1T lasted longer as a result of the necessarily longer TR for the echo-planar images for fMRI and longer scanning times for the T2* map without parallel imaging. However, because the motor and affective paradigms were scanned in random order on both scanners, we are confident that this issue has not confounded our results. Finally, this study included only healthy subjects and not subjects who were obese or claustrophobic, patient groups for whom the open 1T MR imaging scanner has been designed among other things. These patient groups may more often show suboptimal image quality due to subject motion or body habitus, though even in these subjects who are highly obese, the effects of subcutaneous fat tissue around the brain on image quality will be small compared with, for example, the abdomen. However, these possible effects should be taken into account in studies with these patient groups.
Conclusions
The present study shows that though fMRI on an open 1T MR imaging scanner is feasible for group studies, this type of scanner is less suitable for single-subject studies. The optimal TE range for fMRI on the open 1T MR imaging scanner is approximately 70 ms. The use of fMRI on an open 1T MR imaging scanner provides research opportunities for studying groups that are otherwise difficult to enroll, such as patients with claustrophobia and obesity.
Footnotes
-
Paper previously presented as a traditional poster at: International Society for Magnetic Resonance in Medicine, May 1–7, 2010; Stockholm, Sweden.
-
indicates article with supplemental on-line table.
References
- Received June 18, 2010.
- Accepted after revision September 9, 2010.
- Copyright © American Society of Neuroradiology