Abstract
SUMMARY: We report 2 fetal MR imaging cases at 22 wkGA with cerebral bright DWI and low ADC, 8 and 19 days after documented fetal death. These observations illustrate that decreased diffusion can be present weeks after injury onset, and its presence cannot be used to time injury onset within 1 week, which could significantly impact determination of the proximate cause of fetal brain injury in future cases.
Abbreviations
- ADC
- apparent diffusion coefficient
- DWI
- diffusion-weighted imaging
- HII
- hypoxic-ischemic injury
- T2WI
- T2-weighted imaging
- TTTS
- twin-twin transfusion syndrome
- wkGA
- weeks gestational age
The use of DWI and the quantitative changes on ADC maps have long played a central role in the detection and quantitative analysis of acute cerebral ischemia in the adult brain.1 DWI and ADC maps have also become the standard of care for detecting acute ischemic injury in the neonate. However, their role in fetal MR imaging in the evaluation of fetal brain injury has yet to be elucidated.
In adult arterial ischemic stroke without reperfusion, ADC values rapidly decrease within minutes of onset, reaching their nadir value between 28 hours and 4 days, with pseudonormalization occurring between 5 and 10 days.2,3 With reperfusion, a secondary ADC decline may be observed within 2–3 hours and for up to 5 days due to delayed brain cell necrosis.4 In perinatal neonate HII, ADC values decrease within hours after birth, reaching their nadir within 2–3 days and pseudonormalizing within 4–8 days.5,6
On the basis of our PubMed literature search, from 1977 (the date of the first human MR imaging) to April 2010, by using the search terms “fetus,” “MR imaging,” “diffusion,” “ADC,” and “ischemia,” there were only 2 reported cases of DWI signal-intensity increase and ADC decrease in fetal MR imaging. Baldoli et al7 reported 1 case of focal ADC decrease in a 33-week fetus with a vein of Galen malformation with unknown timing of injury onset. Righini et al8 reported 1 case of focal ADC decrease in a 22-week surviving fetus 2 days after co-twin death in the setting of TTTS.
Case Reports
Case 1
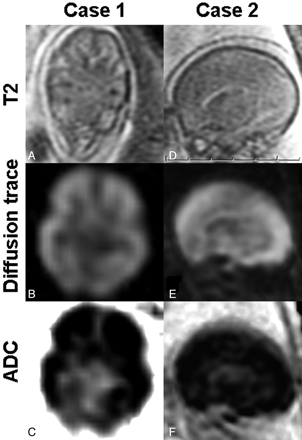
A 33-year-old woman (gravida 4 para 3) with a monochorionic diamniotic twin gestation complicated by TTTS had sonographic documentation of in utero death of one of the twins at 19 + 4/7 wkGA. At 22 + 2/7 wkGA, 19 days after the twin death, a fetal MR imaging with DWI (TR = 2000 ms, TE = 148.1 ms, matrix = 144 × 144, FOV = 40 × 46.1 cm, 7-mm section thickness, b-value = 1000, six directions) was performed. In the deceased donor twin, T2WI showed diminished cortical plate, intermediate zone, and germinal matrix differentiation (Fig 1A) in contrast to the live surviving recipient twin with preserved differentiation of structures (not presented). DWI showed diffuse cerebral bright DWI and low ADC in the deceased twin (Fig 1B, -C). In contrast, the surviving twin showed regional high DWI and low ADC, consistent with normal germinal matrix (not shown).
Axial (case 1) and sagittal (case 2) fetal brain MR images obtained 19 and 8 days, respectively, after confirmed fetal death. Images from both deceased donor twins show diminished cortical plate, intermediate zone, and germinal matrix differentiation on T2WI (A and D) and diffusely high DWI (B and E) consisting of low ADC value signals (C and F).
Case 2
A 39-year-old woman (gravida 1 para 0) underwent an umbilical cord ligation procedure of the donor twin of a monochorionic diamniotic twin pair with TTTS at 21 + 1/7 wkGA. Death of the donor was confirmed by sonography after the procedure. At 22 + 2/7 wkGA, 8 days after the procedure, a fetal MR imaging with DWI (TR = 7000 ms, TE = 90.2 ms, matrix = 128 × 128, FOV = 30 × 34.6 cm, 5-mm section thickness, b-value = 650, six directions) was performed. In the deceased twin, T2WI showed diminished cortical plate, intermediate zone, and germinal matrix differentiation (Fig 1D). DWI showed diffuse cerebral bright DWI and low ADC in the deceased twin (Fig 1E, -F).
Discussion
We present 2 cases of fetal death showing high DWI signal intensity and low ADC values, which persisted far beyond the time period typically observed in adult and neonatal arterial ischemic brain injury, at least 19 and 8 days from sonographically documented fetal death to the follow-up MR imaging. The above-mentioned PubMed literature search from 1977 to April 2010 shows that, to our knowledge, this is likely the first case report documenting decreased brain ADC values more than 1 week after fetal brain injury. This observation suggests that ADC value changes in the fetal brain may have a different time course than those in the neonate or adult.
As reviewed above, in neonatal HII, ADC decreases typically resolve sooner than the time course shown in the 2 cases presented here. Adult stroke evolution is typically faster than neonatal HII, due to the dominance of acute rather than delayed necrosis. The reasons for the timing (between 8 and 19 days) of ADC decreases in the 2 cases presented here remain unclear. Our observation suggests that the evolution of global arterial ischemic brain injury in the human fetus may have a different pathologic and radiologic evolution compared with near-term/term neonates and adults. Differences in etiologic mechanisms (extrinsic factors) or age-specific differences in injury evolution or duration (intrinsic factors) could contribute to these differences.
Extrinsic factors that may result in relatively prolonged ADC decreases in the fetus include a series of recurrent insults in fetal HII as opposed to a single crucial event. Possible obstetric causes of recurrent fetal brain ischemia include cardiovascular instability in TTTS, the fetal immune response to chorioamnionitis, and the fetal circulatory adaptation to uteroplacental insufficiency. In addition, the recurrent insult may trigger secondary energy failure, which could manifest as a delayed ADC decrease. It could also be postulated, in cases of monochorionic pregnancy with placental sharing and vascular communication between the twins, that passive residual circulation provided by the surviving twin could affect the timing of ADC changes. However, in the case of umbilical cord ligation, the presence of residual circulation is exceedingly unlikely and would be plausible only if there was recanalization or incomplete ligation of the umbilical vessels.
Intrinsic factors, such as the time course for tissue necrosis, may be different in the fetal brain. At 22 wkGA, the fetal brain may be more resistant to HII; therefore, the duration of hypoxia and ischemia may need to be much longer before the onset of necrosis. In addition, the normal processes regulating cell death are not fully mature in the fetus; therefore, delayed cell-death mechanisms may need a longer time to activate. These processes have a major pathologic contribution to ischemia in the neonatal and pediatric brain.9 Immaturity of the premyelinating oligodendrocytes and microglias and their inability to mount inflammatory and tissue digestive responses to the primary injury may also affect ADC time course.10
Although a definitive causative explanation for prolonged fetal brain injury after death is not possible without pathologic correlation, this finding is concerning and suggests that our understanding of the pathologic response and natural course of fetal brain injury is incomplete. As we report here, the decrease in fetal brain ADC values can persist for as long as 3 weeks, longer than has been observed in neonates or adults with arterial ischemic brain injuries.
In summary, bright DWI and markedly decreased ADC values in a fetal brain MR imaging do not reliably imply that injury began within 1 week before imaging. This conclusion suggests the need to exercise caution in interpreting diffusion abnormalities in the fetal brain when attempting to determine the timing and the proximate cause of injury.
References
- Received April 22, 2010.
- Accepted after revision April 24, 2010.
- Copyright © American Society of Neuroradiology