Abstract
SUMMARY: Transient CC involvement has been reported in encephalopathies/encephalitis of different etiologies. Here we report 2 patients with AFE, who showed transient pancallosal involvement with restricted diffusion on neuroimaging. Both patients had excellent clinical outcomes: The lesion disappeared completely in 1, though there was mild residual gliosis in the other. Serology for dengue virus was positive in 1 of the patients.
Abbreviations
- ADC
- apparent diffusion coefficient
- AESD
- acute encephalopathy with biphasic seizures and late reduced diffusion
- AFE
- acute febrile encephalopathy
- CC
- corpus callosum
- DWI
- diffusion-weighted imaging
- FLAIR
- fluid-attenuated inversion recovery
- MERS
- mild encephalitis/encephalopathy with a reversible splenial lesion
- NAA
- N-acetylaspartate
Acute encephalopathy is a common clinical scenario in which patients exhibit features of global cerebral dysfunction without a documented central nervous system infection. Etiologies can be diverse, from systemic infections to drugs and metabolic causes. Two patterns on neuroimaging previously reported in this setting are transient splenial lesions with restricted diffusion1–3 and bihemispheric involvement.4 Here we report 2 patients who presented with AFE, who had similar neuroimaging findings of pancallosal involvement with restricted diffusion, 1 of which was initially considered a glioma.
Case Reports
Case 1
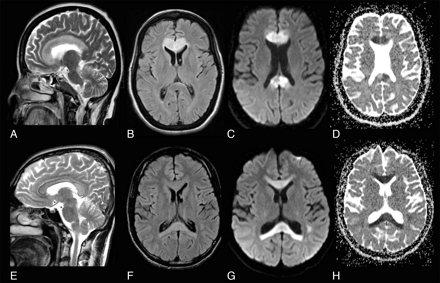
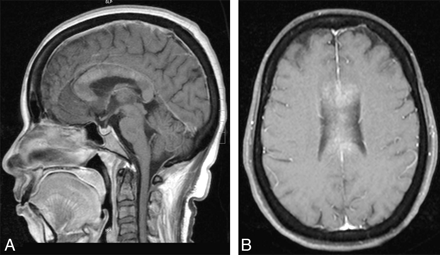
A 45-year-old woman with diabetes mellitus presented to a local hospital with a 1-week history of fever and skin infection, followed by altered sensorium and gait unsteadiness. Her metabolic parameters were normal. She underwent MR imaging in the second week of illness, which showed a pancallosal lesion with restricted diffusion (Fig 1A–D) and contrast enhancement (Fig 2A, -B). With treatment of the infection, her sensorium improved and she was referred to us on day 14 of her illness with a diagnosis of glioma and recommended biopsy.
Case 1. Sagittal T2-weighted (A) and axial FLAIR (B) MR images show pancallosal edema and hyperintensity. C and D, Axial DWI and ADC map show restricted diffusion in the whole of callosum. Case 2. Sagittal T2-weighted (E) and axial FLAIR (F) images show pancallosal hyperintensity. G and H, Axial DWI and ADC map show restricted diffusion in the callosum.
Case 1. Sagittal (A) and axial (B) T1-weighted postcontrast images show enhancement of the callosum.
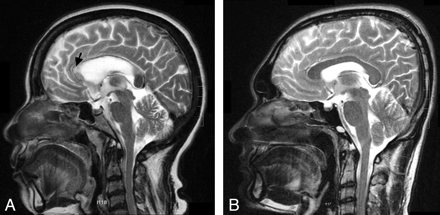
At presentation, the patient was alert and afebrile, with a healing skin ulcer. Findings of a neurologic examination were unremarkable. She had no history of seizures or antiepileptic treatment before admission. Metabolic and hematologic parameters were normal. Additional imaging showed reduced choline and NAA on proton MR spectroscopy, restricted diffusion, and low perfusion on the perfusion imaging, which raised doubt concerning the diagnosis. Biopsy was deferred. She was treated with a short course of steroids. MR imaging repeated 6 weeks later showed near-complete resolution of the lesion with gliosis in the genu and thinning of the CC (Fig 3A). She had no neurologic deficits on follow-up.
A, Sagittal T2-weighted image performed after 6 weeks shows complete resolution of the pancallosal edema. There is evidence of thinning of the callosum with hyperintensity (arrow), suggesting callosal gliosis. B, Sagittal T2-weighted image of the patient obtained after 2 weeks shows complete resolution of the pancallosal hyperintensity.
Case 2
A 27-year-old man presented to us with fever associated with headache and erythematous rash over his body. On day 5 of illness, he had a transient alteration in the sensorium with mild left-arm weakness, which spontaneously subsided in 30 minutes. On day 6 of his illness, he had 2 similar attacks without any preceding convulsive movements. Clinical examination revealed a drowsy patient with subtle left-arm weakness.
Hematologic and biochemical parameters and CSF and electroencephalogram findings were normal. His neuroimaging showed a pancallosal lesion extending to the centrum semiovale symmetrically with restricted diffusion (Fig 1E−H) without contrast enhancement. Serology for dengue was positive. He was observed in a ward without any specific medications. A repeat MR imaging after 2 weeks showed complete resolution of the lesions (Fig 3B). The patient was asymptomatic on follow-up.
Discussion
Prevalence of CC lesions has been reported at 3% in a large series of 450 patients undergoing MR imaging.1 While the CC can be involved in vascular, traumatic, demyelinating, neoplastic, infective, and degenerative illnesses,2 transient involvement has been reported with encephalitis of different etiologies,3,5 antiepileptic drug use and a peri-ictal state. Among infectious etiologies, influenza has been most often associated with transient splenial involvement.6,7
Tada et al8 proposed the terminology “MERS” to explain a clinical syndrome sharing common features of mild encephalopathy, seizures, and good outcome on follow-up. However, in all these reports, the involvement was restricted to the splenium, with some patients having adjacent white matter involvement as well.
Takanashi9 described another entity called “AESD” in children. Neuroimaging showed reduced diffusion in the frontal or frontoparietal subcortical white matter during days 3–9, which reversed on follow-up. Okumura et al4 described 2 patterns of lesions in AESD, a diffuse pattern with poor outcome and a central sparing pattern, which was clinically mild. None of their patients had CC involvement.
Both of our patients had similar clinical presentations, with a background of a febrile illness. The presentation with encephalopathy and minimal focal signs within the first week of illness and symmetric involvement of the CC makes postinfectious demyelination less likely. This clinical pattern has been described in most of the previously reported cases of MERS.3,6–8
MR imaging showed pancallosal involvement with restricted diffusion in both of our patients. The first patient had a bulky CC, with patchy contrast enhancement, which was initially mistaken for glioma. The most consistently reported pathophysiologic mechanisms for CC involvement with restricted diffusion are transient intramyelinic edema and inflammatory infiltrates.10 Some of the recent reports of MERS have shown persistence of lesion/gliotic changes on follow-up, as in our first patient.5,11
Imaging characteristics of neurodengue have rarely been reported, to our knowledge. In a large series of neurodengue,12 no characteristic imaging pattern was noted. A similar observation was made by Wasay et al13 in their series of 6 patients. Their reported findings were cerebral edema and cortical and cord hyperintensities. Even though the findings of callosal hyperintensity with restricted diffusion have never been reported with neurodengue, we presume that the imaging finding may be related to AFE syndrome rather than the virus per se. More imaging studies on neurodengue would be required to clarify the observation.
We report these 2 cases to highlight a few points. Transient pancallosal involvement with restricted diffusion is a novel observation in AFE of any etiology. It may represent an extended spectrum of MERS because the presentation and outcome were similar. Encephalopathy associated with dengue has been linked to a poor outcome as opposed to our patient who had mild symptoms, which resolved spontaneously. Last, the appearance of the lesion can be mistaken for a glioma because of the associated swelling and contrast enhancement. However, the clinical setting and additional MR imaging sequences, including diffusion and perfusion, can differentiate the 2, thereby avoiding invasive biopsy.
References
- Received July 20, 2010.
- Accepted after revision July 31, 2010.
- © 2011 by American Journal of Neuroradiology