Abstract
BACKGROUND AND PURPOSE: tSCH in the absence of spinal trauma or surgery is a rare disorder for which numerous mechanisms have been proposed. Here, we have conducted an analysis of images in all published reports of idiopathic tSCH and identified evidence supporting a pathogenesis in which anterior dural erosion at thoracic levels generates a CSF leak that pushes adjacent spinal tissue to tamponade the dural defect, causing progressive myelopathy. Additionally, we describe a case of tSCH in which postural headache was a significant symptom before myelopathy. This finding suggests that tSCH pathogenesis may be related to spontaneous intracranial hypotension.
MATERIALS AND METHODS: Published imaging from all available prior case reports in the scientific literature was reviewed to determine whether tSCH occurred at the disk or bone level. The presence of EDF, HNP, or an osteophyte in the spinal canal was determined from review of published images. Additionally, 3 previously unreported cases from the teaching files of our department were assessed by using these criteria.
RESULTS: In greater than two-thirds (47 of 67) of identified cases with published images, tSCH occurred at a disk level. When assessment was possible, EDF, HNP, and osteophytes were present in 26.8%, 30.7%, and 26.2% of cases, respectively. Overall, 52.3% of cases with published images demonstrated evidence of these abnormalities.
CONCLUSIONS: Our analysis of published imaging indicates that tSCH occurs preferentially at spinal levels and with imaging findings consistent with dural injury that support the proposed etiology of this disorder.
Abbreviations:
- EDF
- extra-dural fluid
- HNP
- herniated nucleus pulposus
- SIH
- spontaneous intracranial hypotension
- tSCH
- thoracic spinal cord herniation
Idiopathic tSCH is a rare disorder typically presenting in middle-aged patients with myelopathy in whom the anterior portion of the spinal cord herniates through a defect in the ventral dura. While spinal trauma or surgery may produce such defects, idiopathic herniation occurs in the absence of this history. Since the initial report by Wortzman et al,1 numerous mechanisms explaining its pathogenesis have been proposed, including minor or unrecognized trauma,2,3 congenital meningeal malformations,4–8 CSF flow pulsations,9,10 and dural erosion by calcified disk remnants.11–15 While >100 cases have been reported, no consensus exists as to the most likely mechanism.
We conducted a retrospective analysis of the imaging published in all available English-language case reports of tSCH. We examined images in these articles for the presence of specific characteristics that, when present, suggest a pathogenesis of tSCH, in which an unrecognized thoracic disk or osteophyte erodes the anterior dural surface permitting normal CSF pulsations7 to propel the cord to tamponade and herniate through the meningeal defect. Along with our analyses, we have chosen 1 recent case from our institution not only to illustrate the proposed pathogenesis but also to highlight a possible association with SIH. Our analyses indicate that in most cases, thoracic cord herniation occurs at a disk level and has at least 1 of the accompanying findings consistent with the suggested pathogenesis.
Materials and Methods
Retrospective Analysis
We examined all English-language case reports in the scientific literature describing idiopathic thoracic spinal cord herniation. Next, we identified applicable studies through referencing recent reviews of the literature and PubMed searches of the data base of the National Library of Medicine of published scholarly articles. Each study was reviewed to identify confirmed cases of thoracic spinal cord herniation without a history of trauma and surgery. Of those cases, ones that included at least 1 image (sagittal and/or coronal planes) at the site of herniation before intervention were included in the analysis. Additionally, we have included 3 recent cases of tSCH from our institution in the analysis. These cases share characteristics similar to those previously reported, with no history of recognized trauma or iatrogenesis.
Each applicable case was analyzed for several characteristics, with assessment in all but 2 cases (On-line Table) based completely on the published images. Initially, the text of the history of each case was examined for notation of headache as a symptom before intervention for spinal herniation. Imaging techniques used to identify spinal herniation were recorded. Next, we analyzed the published images for the following characteristics: 1) the point of herniation relative to disk level, 2) the presence of EDF, and 3) the presence of disk protrusions or osteophytic spurs near or into the dura.
Using available sagittal spine images, we categorized the herniation point as occurring either at the intervertebral disk or vertebral body level. Typically, a herniated spinal cord tents ventrally through a discrete dural fistula adjacent to the dorsal surface of the vertebral column. In images in which there was a wide area of tenting spanning both the disk and bone level, the midpoint of the herniating spinal cord was determined to be the tent point. When the point or midpoint of herniation occurred at the border between the disk space and the vertebral body, the following technique was used to confirm its position: Using a photo-processing software, Photoshop (Adobe Systems, San Jose, California), we imported the image, over which a line was drawn perpendicular to the rostrocaudal axis of the spinal cord connecting the posterior edge of the vertebral body to the anterior edge of the spinous process at the same spinal level.
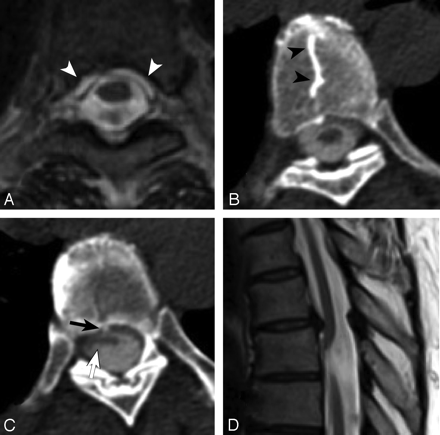
We assessed available axial images for the presence of EDF, evidence of HNP, and the presence of an osteophyte emanating from the dorsal surface of the vertebral body at or near (within 1 spinal level) the point of cord herniation (Fig 1D). Tallies of the cases with each of the 4 characteristics (herniation point, EDF, HNP, osteophytes) were created. Descriptive statistics were calculated for each category for all cases and for subgroups of cases with published sagittal and axial images for the case.
Axial T2-weighted MR image (A) and contiguous axial sections from a CT myelogram (B and C) demonstrate ventral extradural CSF (A, white arrows) and a thoracic “nuclear cleft” (B, arrowheads) through which a herniated disk protrudes into the ventral dura (C, arrows) and extradural contrast is noted along with a ventrally herniated spinal cord (C). D, Sagittal T2-weighted image at the upper thoracic spinal cord shows typical ventral herniation of the cord inferior to a posteriorly directed osteophyte.
On CT myelography, breach of the dural sac permits radiodense CSF to extravasate around the dural sac and into the adjacent epidural space (Fig 1A). Fluid extravasation into this space may also appear as an interruption of the normally smooth round shape that contours the subdural space. In those cases with published axial images from CT myelography, we deemed EDF to be present when irregularity of this contour was present and/or radiodense signal intensity was seen in the adjacent extradural space.
Evidence of HNP included the characteristic radiographic finding of a calcified “nuclear trail” along the superior or inferior surface of adjacent vertebral bodies associated with a soft-tissue component of herniated disk (Fig 1B). Seen on axial CT, a nuclear trail appears as an abnormal straight or curvilinear attenuation positioned along the midline of the vertebral body and has been found to be present in >40% of confirmed thoracic disk herniations. There is an MR imaging correlate, a “comet tail,” which is similarly positioned and occurs at a similar rate in thoracic disk herniations. The presence of the nuclear trail represented the sole evidence used in this study to identify thoracic disk herniation.16
Osteophytes were deemed present if a thin elongated hyperattenuated spicule of bone protruded from the posterior surface of the vertebral body toward the anterior dural surface (Fig 1C). Axial sections were closely examined for the presence of a small area of hyperattenuated signal intensity at the midline of the dorsal surface of the vertebral body, jutting posteriorly. The hyperattenuated area was considered thin if it was no wider than an adjacent nuclear trail (see above) or a visually predicted one if not present. Additionally, the osteophyte must have disrupted the predicted smooth contour of the posterior surface of the vertebral body. In cases in which no consensus was reached, the case was excluded from the HNP calculation.
Results
Retrospective Analysis
Our analysis of published imaging in 44 scholarly articles identified 67 distinct cases of idiopathic thoracic spinal cord herniation. Three previously unreported cases from our teaching file data base were added, making a total of 70 evaluable cases (On-line Table). In all cases, sagittal spine imaging had been published, allowing determination of the level of spinal cord herniation. Greater than two-thirds (67.1%) of herniations occurred at the disk level. Headache preceding the myelopathy associated with tSCH was reported in only 1 case (of 67) in the literature6 and in 2 of the 3 cases from our teaching file.
Axial spine imaging of sufficient clarity to evaluate EDF, HNP, and osteophytes was available for review in approximately two-thirds (53 cases) of the 70 cases. In the subset of cases permitting axial spine assessment, there was evidence of EDF in 28.9% (n = 13 of 45), HNP in 30.2% (n = 13 of 43), and posteriorly directed osteophytes in 29.8% (n = 13 of 47) at the site of the spinal cord herniation. In most cases in which EDF (n = 9 of 14), HNP (n = 7 of 13), or osteophytes (n = 10 of 14) were identified (on the axial image), the cord herniation point was at the disk level. Overall, more than half (52.3%, 23 of 44) of cases had evidence of EDF, HNP, or osteophytes, with a higher rate (59.3%, 16 of 27) among those cases in which the herniation point occurred at the disk level.
Case Report
A 53-year-old woman initially presented 20 years before her present admission with an episode of intense postural headache that resolved after 2 weeks of bed rest in the supine position. Six months later, she developed sensory changes in her right leg and subsequent myelopathy that progressed in severity for several years. In 2005, five years before her present admission, the patient was diagnosed with thoracic cord herniation on MR imaging and underwent 2 surgeries to release her thoracic spinal cord, which was strangulated through a ventral dural defect at the thoracic level, which was repaired with a bovine graft. Despite these procedures, the patient continued to have progressive numbness, tingling, and weakness in her lower extremities, with bowel and bladder dyssynergia. MR imaging of the spine showed progressive edema of the spinal cord, consistent with presyrinx. Myelography at that time showed a blockade of CSF flow secondary to arachnoid adhesions at the point of her prior surgery (Fig 1B, -C).
Discussion
In this study we have addressed the pathogenesis of idiopathic tSCH through retrospective analysis. Coupled with this analysis is the description of 1 of our patients with MR imaging and surgically documented tSCH in whom severe postural headache, the cardinal symptom of SIH, resolved before she developed progressive myelopathy and imaging findings consistent with tSCH. The results of the retrospective analysis support the proposed pathogenesis that unrecognized trauma by herniated disk material or endplate osteophytes to the anterior dural surface in the thoracic spine leads to eventual herniation of the thoracic spinal cord through a dural defect. The report illustrates this pathogenesis and suggests a link to SIH.
Our analysis is consistent with the theory that a herniated thoracic disk or osteophyte pierces, lacerates, or progressively erodes the anterior thoracic dura. This defect permits CSF extravasation, which may resolve with time, to continue if untreated and result in SIH, or become tamponaded by the spinal cord. In greater than two-thirds of cases we reviewed, the apex of the spinal cord herniation point occurred at a disk level. We identified disk osteophytes in nearly one-third of cases, despite the fact that nearly all analyzed cases published only a single cross-section (at the point of herniation) of the rostrocaudal length of the thoracic spinal column.
The apparent inconsistency between the rate of disk level herniation and the rate of disk osteophytes can be attributed to significant motion of the spinal cord dura relative to the spinal column during flexion and extension movements. Thus, it is likely that the structure puncturing and/or lacerating the dural surface lies rostral or caudal to the site of the published cross-sectional image. This implies that the presence of disk osteophytes in the reviewed cases may be significantly under-represented. Assessment of extradural fluid was similarly limited by the amount of cross-sectional imaging, yet it too was present in almost one-third of cases, suggesting that extravasation of CSF through and/or around the dural defect continues despite the herniation of the spinal cord. If additional cross-sectional imaging immediately above and below the herniation point had been available, we presume that the rate of this finding would have been significantly greater.
The upper thoracic cord is a likely place for the anterior dural surface to be breached by calcified disk remnants because it is the spinal cord region most closely apposed to the posterior surface of the vertebral bodies and intervertebral disks. Barbagallo et al11 reported that spinal cord herniation has never been reported at other spinal regions unless there was a history of significant trauma or iatrogenic injury. The relative positions of the spinal cord and dura change with flexion and extension of the spinal column. Such movement may permit osteophytes to contact and lacerate the dura. CSF may then exit the dura and migrate rostrally or caudally before the spinal cord herniation tamponades the defect. Consistent with the proposed pathogenesis, evidence of an HNP at the level of the thoracic vertebrae was present in >30% of cases we reviewed. Asymptomatic thoracic disk herniation has an estimated incidence of 11.1%–13.3%,17 whereas disk herniation at this level producing symptoms has an overall incidence of 1 per 1 000 000 patient-years.18 Most interesting, thoracic disk herniation has a high degree of calcification of herniated disk elements, and the herniations occur almost exclusively in the ventral or ventrolateral direction.18 Given the incidence of asymptomatic herniations at this level, we suspect that the recorded percentage of HNP would be significantly greater if additional cross-sectional imaging was available.
Our case report suggests our proposed pathogenesis of tSCH and indicates a possible connection between tSCH and SIH. The patient presented developed a progressive myelopathy that, as others have reported, develops in the absence of known trauma or spinal manipulation. However, our patient reported a severe but transient postural headache that spontaneously resolved years before the development of myelopathy. Severe postural headache is 1 of the earliest and most prominent symptoms of intracranial hypotension, a disorder that occurs following therapeutic or traumatic dural perforation and subsequent leakage of CSF.19 In a small portion of cases, the symptoms develop spontaneously without known provocation; the primary defect is the loss of CSF volume through spinal CSF leaks.20
From the proposed pathogenesis of tSCH and the case reported, we hypothesize that the tamponade of a dural defect by the spinal cord prevents or ameliorates the development of SIH. Once the dural defect is created, extravasation of CSF may continue until the fistula closes spontaneously or is closed by surrounding tissue or by a primary dural repair. Given CSF flow patterns, the overlying arachnoid may develop adhesions with the dural defect.7 Subsequently, the spinal cord or nerve roots may tamponade the dural defect and prevent further CSF outflow. If closure of the defect does not occur, continued loss of CSF volume may be sufficient to result in intracranial hypotension and the associated postural headache. By proposing a potential connection between tSCH and SIH, we hope not only to better understand their pathogenesis but also to increase clinical recognition of these syndromes.
During our review of previously published cases of tSCH, we found only 1 other mention of postural headache in the clinical history.6 An explanation of this absence is that headache in the setting of tSCH may have been regarded by authors as a nonspecific symptom with no association to the primary disorder. Conversely, this may reflect a lack of inquiry about this symptom or a delay between the resolution of headache and the development of SIH. As with our patient, the time between the SIH symptoms and tSCH-related myelopathy may be long enough that the patient fails to recall it or the physician fails to elicit the history. Indeed, only after close questioning did our patient describe postural headaches 20 years preceding the development of myelopathy due to thoracic cord herniation. This patient illustrates the temporal dissociation between resolution of the headache and the often insidious onset of the myelopathy secondary to spinal cord herniation. If our proposed pathogenesis of tSCH is correct, unreported headache in prior cases may also simply reflect the transient nature of headache as a symptom.
Alternative explanations for the pathogenesis of tSCH rely on congenital malformations of the spinal meninges. Duplication of dura21 and arachnoid cysts have been suggested in several cases as the cause of cord herniation and strangulation. If correct, one might expect these malformations to be scattered throughout the rostrocaudal extent of the spinal cord and to occur in all age groups. However, our review of the published literature agrees with the assertion by others11 that there are no reports of idiopathic cord herniation at other spinal levels. In a recently published case report, CSF kinetic studies excluded the possibility of a posterior arachnoid cyst.22 This finding suggests that the presence of an “arachnoid cyst” identified on static imaging modalities may be an incorrect interpretation of enlarged posterior CSF spaces due to ventral tenting of the cord. Additionally, in a review by Pereira et al,23 arachnoid cysts were present in only one-quarter of cases. These authors also reviewed several cases with evidence of meningeal inflammatory changes consistent with prior minor trauma, a pathogenesis proposed by Tronnier et al.3 The apparent restriction of cord herniation to the thoracic region thus points more strongly toward mechanical and anatomic factors specific to the thoracic cord.
Therapeutic implications of an identified mechanism for tSCH are significant. If tSCH develops according the proposed pathogenesis, then the symptom of postural headache that resolves spontaneously may serve as a harbinger for later development of severe focal neurologic sequelae. As noted in the case presented above, the SIH-like syndrome may precede the development of progressive myelopathy. The link to SIH, an infrequent but not rare disorder,19 implies that SIH and tSCH represent 2 different sequelae of a spontaneous breach of the spinal dura. In many patients, dural punctures or lacerations can be readily treated with a minimally invasive epidural blood patch, a procedure that provides almost instantaneous relief.19 Because the standard treatment for tSCH is laminectomy with open neurosurgical reduction of the herniated spinal tissue and dural repair, the consequences of delayed recognition are significant to treatment and patient recovery. In addition, follow-up imaging might be considered in patients presenting with resolution of SIH secondary to thoracic ventral leaks due to osteophytes or HNP. Although myelography was formerly required to identify tSCH, the advent and widespread use of high-resolution MR imaging provides a readily available alternative with comparatively few technical requirements.
Conclusions
Ventral thoracic spinal cord herniation is a rare disorder that remains without a clearly identified pathogenesis in most cases. Given the increasing body of cases reported in the literature, we have assessed the frequency of specific imaging characteristics present in the published imaging of these idiopathic cases. We believe that in most cases, the pathogenesis of tSCH is initiated by incidental or unnoticed trauma to the anterior surface of the thoracic dura by disk protrusions or osteophytes. Subsequent herniation of spinal cord tissue through a dural fistula results from the action of CSF pulsation and mechanical factors, aided perhaps by arachnoidal adhesions, which lead to progressive myelopathy. This hypothesis also is consistent with the pathogenesis of some cases of SIH, which is illustrated in our “Case Report.” If confirmed, the proposed pathogenesis of tSCH and its link to SIH have significant diagnostic and therapeutic implications.
Acknowledgments
We acknowledge the contributions and advice of the following individuals with regard to the preparation of this manuscript: Mauricio Castillo, MD, Michelle Micheal, MD, and Douglas Phillips, MD.
References
- Received April 3, 2011.
- Accepted after revision May 15, 2011.
- © 2012 by American Journal of Neuroradiology