Abstract
BACKGROUND AND PURPOSE: IIH is a syndrome of elevated intracranial pressure without hydrocephalus, mass, or identifiable cause. Diagnosis is made by clinical presentation, intracranial pressure measurement, and supportive imaging findings. A subset of patients with IIH may have tonsillar ectopia, meeting the criteria for Chiari malformation type I but not responding to surgical decompression for Chiari I. The purpose of this study was to determine the incidence and morphology of cerebellar tonsillar ectopia in patients with IIH.
MATERIALS AND METHODS: Forty-three patients with clinically confirmed IIH and 44 age-matched controls were included. Two neuroradiologists with CAQs reviewed sagittal T1-weighted MRI in a blinded fashion and measured cerebellar tonsil and obex positions relative to the foramen magnum and prepontine cistern width at the level of the midpons.
RESULTS: Nine of 43 patients with IIH and 1/44 controls had cerebellar tonsillar ectopia of ≥5 mm. Five of 9 of patients with IIH with ectopia of ≥5 mm also had a “peglike” tonsil configuration. Patients with IIH had a significantly lower tonsillar position (2.1 ± 2.8 mm) than age-matched controls (0.7 ±1.9 mm, P < .05). The obex position was significantly lower in patients with IIH versus controls (−7.9 mm [above the FM] versus −9.4 mm [above the FM], P < .05). The prepontine width was not significantly different between the groups.
CONCLUSIONS: Cerebellar tonsil position in patients with IIH was significantly lower than that in age-matched controls, often times peglike, mimicking Chiari I. A significantly lower obex position suggests an inferiorly displaced brain stem and cerebellum. When tonsillar ectopia of >5 mm is identified, imaging and clinical consideration of IIH are warranted to avoid misdiagnosis as Chiari I.
ABBREVIATIONS:
- CAQ
- Certificate of Added Qualification
- CM
- Chiari malformation
- FM
- foramen magnum
- ICP
- intracranial pressure
- IIH
- idiopathic intracranial hypertension
IIH, previously known as pseudotumor cerebri, is a syndrome characterized by elevated ICP with normal CSF composition and no other identifiable cause.1 It has been proposed that the elevated ICP may be related to decreased CSF resorption due to impaired venous outflow and elevated venous pressure; however, controversy still surrounds the significance of venous sinus stenosis in IIH as the cause or the result of elevated ICP.2 IIH predominantly affects young overweight (body mass index >25) women with a reported incidence of 19/100,000 in this population.2,3 Patients with IIH most commonly present with headaches, occurring in 68%–98%.2,4 Other clinical features include pain, pulsatile tinnitus, and visual disturbance, which can lead to blindness.2,5 Treatment consists of weight reduction, acetazolamide, and surgical intervention, including CSF shunt surgery.6⇓–8
Although IIH is a clinical diagnosis based on normal CSF composition with an elevated opening pressure (>20 cm H2O in nonobese patients and >25 cm H2O in obese patients with body mass index >30), supportive neuroimaging findings have been described. These include flattening of the posterior sclera, tortuosity of the optic nerve sheath, empty sella syndrome, and stenosis of the transverse venous sinuses.9,10 Therefore, imaging can aid in making or supporting the clinical diagnosis in some cases, especially if clinicians are not as familiar with the diagnosis. The incidence and morphology of cerebellar tonsillar ectopia in IIH has not been previously described in the radiology literature, to our knowledge. When present, tonsillar ectopia in IIH may confuse the radiographic picture and mimic other entities more commonly associated with tonsillar ectopia, such as Chiari I malformation and spontaneous intracranial hypotension.
Chiari I malformation is characterized by caudal protrusion of “peg-shaped” cerebellar tonsils below the foramen.11,12 Chiari I malformation is defined radiographically as an inferior displacement of the cerebellar tonsils of ≥5 mm below the opisthion-basion line.13,14 In the healthy adult, cerebellar tonsils are rarely >3 mm below the foramen magnum. Patients with the radiographic appearance of Chiari I malformation can be asymptomatic, but the most common clinical symptoms include headache, neck pain, vertigo, sensory changes, and poor coordination. Therefore, clinical symptoms may overlap IIH.11 Chiari I malformation is also associated with abnormal CSF flow, which can lead to syringomyelia. Treatment of Chiari I consists primarily of surgical hindbrain decompression with suboccipital craniectomy to restore normal flow at the foramen magnum.15
Previous studies in the surgical literature describe a subset of pretreatment patients with IIH with cerebellar tonsillar ectopia, meeting the criteria for Chiari I.16⇓⇓–19 Fagan et al18 described a “Chiari pseudotumor cerebri syndrome” to highlight the coexistence of Chiari I and IIH in some patients and the difficulty in treatment. Most of these patients with IIH and presumed Chiari I were initially treated with surgical decompression with no clinical improvement (Fig 1). However, many ultimately did respond to CSF shunt surgery, one of the treatments for IIH.18 There is evidence in the clinical and surgical literature that Chiari and IIH may coexist. A previous study by Banik et al20 observed tonsillar ectopia of >2 mm in 24% of patients with IIH and tonsillar ectopia of ≥5 mm (radiographic criterion of Chiari I) in only 11% of patients with IIH. Another study by Johnston et al21 found only 6% of patients with IIH with radiographic criteria of Chiari I. Unfortunately, a cause and effect relationship has not been proved. In other words, it is not clear whether patients with congenital Chiari I develop elevated intracranial pressures and a secondary diagnosis of IIH or whether patients with IIH secondarily develop tonsillar ectopia, which may be mislabeled Chiari I. Therefore, treatment considerations may be complex. Regardless of the cause and effect relationship, it has been shown that patients with IIH may be classified and treated as having Chiari I and may undergo surgical hindbrain decompression, yielding little to no benefit in this subset of patients.18,22
A 43-year-old woman initially diagnosed with Chiari I and treated with surgical decompression. The patient had persistent headaches and recurrent pseudomeningoceles at the surgical site. The patient was ultimately diagnosed with IIH and underwent ventriculoperitoneal shunt surgery with symptomatic relief. A, Sagittal T2-weighted image shows a peglike herniation of the cerebellar tonsils (arrow). However, a partially empty sella is also noted (arrow), which could have been a clue to the underlying or coexistent IIH. B and C, Sagittal and axial T2WI shows the postsurgical changes from suboccipital craniectomy and a complex extracranial fluid collection, compatible with pseudomeningocele (arrow).
This study aimed to determine the incidence and morphology of cerebellar tonsillar ectopia in patients with IIH to further clarify the relationship between a clinical diagnosis of IIH and the presence of cerebellar tonsillar ectopia of ≥5 mm. This study emphasizes the importance of clinical history and supplementary radiographic evidence to distinguish cerebellar tonsillar ectopia of ≥5 mm in the setting of IIH from the “typical” Chiari I.
Materials and Methods
Patient Population
After obtaining institutional review board approval, a retrospective search for the terms “idiopathic intracranial hypertension” or “pseudotumor cerebri” in MR imaging reports from 2008 to 2010 yielded 90 patients. After a comprehensive chart review, only 46 patients (mean age, 36 ± 12 years; range, 18–61 years; 45 female) had a clinically confirmed diagnosis of IIH with ICP measurements (>20 cm H2O for nonobese patients and >25 cm H2O for obese patients). An inclusion criterion was also MR imaging with a sagittal T1-weighted sequence. Three female patients were excluded because MR images did not include sagittal T1-weighted images. Forty-four age-matched control patients were selected (mean age, 40 ± 12 years; range, 18–61 years; 27 female).
Image Review
Two neuroradiologists with CAQs reviewed selected sagittal T1-weighted MR images of the 43 patients with IIH and 44 age-matched controls in a blinded fashion. The cerebellar tonsil and obex positions relative to the foramen magnum were measured by drawing a line from the basion to the opisthion to define the plane of the foramen magnum.23 If the tonsils were above this reference line, the measurement was assigned a negative number; if the tonsils were below the line, the measurement was a positive number. All obex positions were above this reference line and were assigned a positive number (Fig 2 ). In addition, the prepontine cistern width at the level of the midpons was measured. Measurements from patients with IIH and control subjects were compared with standard 2-sample t tests, assuming equal variances. P values <.05 were considered statistically significant.
Cerebellar tonsil, obex position, and prepontine cistern width measurements. A, Sagittal T1-weighted image shows the foramen magnum reference line from the opisthion to the basion. Note the appearance of the obex (arrowhead) and the large empty sella in this patient with IIH (arrow). B, Sagittal T1WI shows the measurement from the obex to the foramen magnum reference line. Note that the obex was above the foramen magnum and would be assigned a negative number (−8 mm). C, Sagittal T1WI shows all 3 measurements in a healthy control. Note that the cerebellar tonsils are above the foramen magnum and would, therefore, be assigned a negative number (−9 mm for tonsil and obex positions). D, Sagittal T1WI shows the measurement from the cerebellar tonsil to the foramen magnum. Note that the cerebellar tonsils are below the foramen magnum and would therefore be assigned a positive number (5 mm).
Results
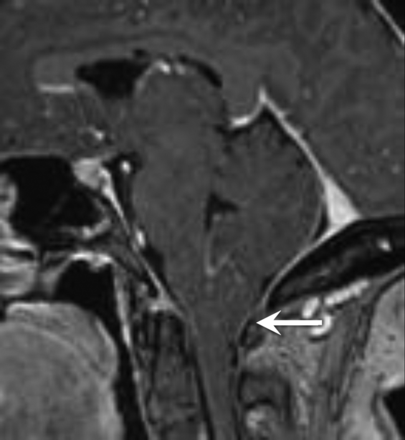
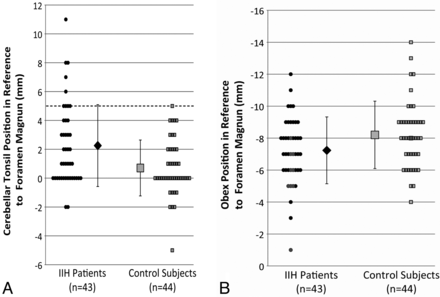
Cerebellar tonsillar ectopia of ≥5 mm was found in 9/43 patients with IIH (20.9%) and in only 1/44 control subjects (2.2%). Five of the 9 patients with IIH with tonsillar ectopia of ≥5 mm also had a peglike configuration of their cerebellar tonsils, closely mimicking CM (Fig 3 ). Of the 9 patients with IIH with tonsillar ectopia of ≥5 mm, 8/9 responded to treatment for IIH alone. One patient ultimately underwent a surgical decompression for CM. Patients with IIH had significantly lower mean tonsillar positions (2.1 ± 2.8 mm) than age-matched controls (0.7 ± 1.9 mm, P < .05) (Fig 4 ). There was no statistical difference in mild cerebellar tonsillar ectopia (2–4 mm) between patients with IIH and controls. Twelve of 43 patients with IIH (28%) and 12 of 44 control patients (27%) had tonsillar ectopia between 2 and 4 mm.
A patient with IIH with tonsillar ectopia of ≥5 mm. Sagittal T1-weighted image demonstrates herniation and a peglike configuration of the cerebellar tonsils below the foramen magnum.
Differences in the tonsillar and obex positions between patients with IIH and healthy controls. Note that negative numbers are above the foramen magnum and positive numbers are below the foramen magnum. A, Differences in the tonsillar position between patients with IIH and control subjects. More than 20% of patients with IIH had cerebellar tonsillar ectopia of ≥5 mm (dotted line) (P < .05), the current criterion for radiologically diagnosed Chiari I malformation. B, The position of the obex relative to the foramen magnum was also significantly different (P < .01) between patients with IIH and control subjects. Black markers in B correspond to subjects with cerebellar tonsillar ectopia of ≥5 mm, indicating that it may be possible for additional discrimination between Chiari I and patients with IIH.
The prepontine cistern width was not significantly different between the 2 groups (P = .3). The mean obex position was significantly lower in patients with IIH versus healthy controls (−7.9 ± 2.8 mm [above the opisthion-basion line] versus −9.4 ± 2.9 mm; P < .05) (Fig 4). When we compared the obex position, the subset of patients with cerebellar tonsillar ectopia in the IIH group also had a significantly lower obex position than in the control patients (−5.6 ± 2.0 mm [above the opisthion-basion line] versus −9.4 ± 2.9 mm; P < .05). Results are also summarized in the Table. Note that negative numbers denote a position above and positive numbers, a position below the opisthion-basion line.
Comparison of measurements from patients with IIH and control subjectsa
Discussion
The cerebellar tonsillar position is below the foramen magnum in Chiari I and may or may not be low-lying in IIH. Cerebellar tonsillar ectopia is not diagnostic of Chiari I only, and one should take care when interpreting cross-sectional imaging because all cerebellar tonsillar ectopia does not equal Chiari I malformation. In this study, a statistically significant number of patients with IIH were found to have tonsillar ectopia of ≥5 mm, mimicking a Chiari I malformation. We found a higher incidence (21%) of tonsillar ectopia of >5 mm in patients with IIH than the 10% previously reported by Banik et al.20 Banik et al used different terminology than the current authors. We have not used the term Chiari I malformation in the setting of IIH. Instead, we have used cerebellar tonsillar ectopia to refer to tonsil position in patients with IIH that may be acquired rather than a malformation but meets the radiographic criteria for Chiari I. We believe that using the term CM in the setting of IIH can be confusing and could even result in inappropriate treatment until a relationship has been proved. Banik et al reported that 24% of patients with IIH had tonsillar ectopia of >2 mm, but only 10% had tonsillar ectopia of >5 mm.20 The difficulty in managing this subset of patients with a diagnosis of IIH and cerebellar tonsillar ectopia of ≥5 mm (meeting the criterion for Chiari I) has been previously reported in the surgical literature, though there is little discussion in the radiologic literature.17,18,20,22
Chiari I is considered a disorder of the paraxial mesoderm with hindbrain maldevelopment and small posterior fossa volume. IIH has evidence of elevated intracranial pressure, altered CSF absorption, and intracranial compliance.21,22 The relationship between these 2 diagnoses has been established but is poorly understood. It is possible that elevated intracranial pressure in IIH may cause cerebellar tonsils to herniate through the foramen magnum, manifesting imaging criteria of Chiari I. Alternatively, it is possible that patients with Chiari I have abnormal CSF dynamics, which predispose to elevated intracranial pressure and IIH. It is postulated that patients with coexistence of Chiari I and IIH may have relief after posterior fossa decompression, which alters compliance. However, symptoms often recur, and these patients with IIH may subsequently require a CSF shunt surgery procedure (Fig 1).
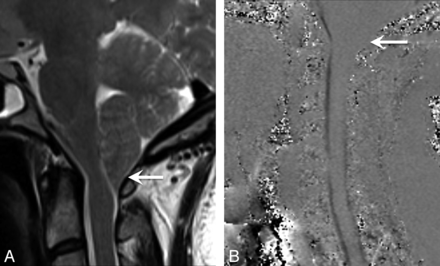
However, the reverse sequence of events has also been reported and was encountered in our cohort. Patients with IIH with cerebellar tonsillar ectopia initially treated with CSF shunt surgery may go on to require decompression. One of the 9 patients with IIH with tonsillar ectopia of ≥5 mm ultimately required a surgical decompression. During the course of 10 years, this patient was treated with both a ventriculoperitoneal shunt and a lumbar peritoneal shunt. Initially, the patient responded well to these treatments with dramatic relief of her IIH symptoms. Her course was complicated by low-pressure headaches treated with a shunt revision, which may have contributed to the appearance of her tonsils. Ultimately, she developed refractory suboccipital headaches that were treated with suboccipital craniectomy. Her surgeon noted that her tonsillar ectopia was not a typical Chiari I but was likely acquired tonsillar ectopia from longstanding IIH and a functioning lumbar peritoneal shunt. Indeed acquired tonsillar ectopia meeting the radiographic criteria for Chiari I and even syringomyelia has been described in patients with IIH after lumboperitoneal shunt surgery.24 Nevertheless, our surgeon concluded that her symptoms and refractory suboccipital headache were due to crowding at the foramen magnum. She underwent surgical decompression for a presumed diagnosis of Chiari I with a complicated course (Fig 5 ).
A patient with IIH with cerebellar tonsillar ectopia of ≥5 mm. This was the only patient with IIH who ultimately underwent surgical decompression after being managed for 12 years with CSF shunt surgery procedures alone. A, Sagittal T2-weighted image demonstrates herniation of the cerebellar tonsils with a peglike configuration and crowding at the foramen magnum. B, Cine CSF flow study shows loss of CSF flow posteriorly at the foramen magnum (arrow).
Although there is no consensus about the ideal management for these patients, it is clear that careful consideration, upfront measurement of intracranial pressure, and inclusion/exclusion of classic IIH are important before surgically treating any patient with Chiari I or IIH with secondary tonsillar ectopia mimicking Chiari I. For these reasons, radiologists can play a critical role in suggesting IIH instead of Chiari I, on the basis of additional imaging features such as transverse sinus stenosis, empty sella, and tortuous optic nerves (Fig 6 ).25
A patient with IIH with tonsillar ectopia of ≥5 mm. Sagittal T1-weighted image shows herniation and a peglike configuration of cerebellar tonsils (arrow).
Patients with IIH also had a significantly lower mean tonsillar position compared with healthy controls, again suggesting that a low cerebellar tonsil position may be acquired in patients with IIH, possibly due to chronically elevated ICP. However, there was no statistical difference in mild tonsillar ectopia (2–4 mm) between the patients with IIH and controls. The incidence of mild cerebellar tonsillar ectopia in our patients with IIH (28%) was similar to that previously reported by Banik et al (24%).20 Unlike the Banik et al, we also compared our patients with IIH with healthy controls. Initially we were surprised that the incidence of mild tonsillar ectopia was similar in our patients with IIH and healthy controls. The reason for this similarity was that the mean tonsillar position in our healthy controls (0.7 ± 1.9 mm below the foramen magnum) was significantly lower relative to the opisthion-basion line than previously reported. Previously Barkovich et al13 and Aboulezz et al14 reported a mean tonsillar position of 1 and 2.9 mm, respectively, above the foramen magnum.
Two other measurements were performed during this study: 1) prepontine cistern width and 2) obex position relative to the foramen magnum. There was no statistical difference in prepontine cistern width between the controls and patients with IIH. The obex position, defined as where the fourth ventricle becomes the central canal of the cervical spinal cord, is a marker for the cervicomedullary junction. The obex position relative to the foramen magnum was found to be significantly different in patients with IIH relative to controls (7.9 ± 2.8 mm above the FM versus 9.4 ± 2.9 mm above FM; P < .01). This difference implies that there is a downward shift of the brain stem in patients with IIH. Patients with Chiari I are expected to have a normal obex position, though there have been reports of mild descent of the fourth ventricle and medulla.26 If one considered only the subset of 9 patients with IIH with tonsillar ectopia of >5 mm, the obex position was even more significantly different from that in control subjects (5.0 mm ± 2.0 mm versus 9.4 mm ± 2.9 mm, P < .005). Hence, it may be possible to select a threshold for the obex position (Fig 4B) that could be used as an additional radiologic finding to separate 2 distinct patient populations that currently overlap. These findings are intriguing, but a future study of the obex position in patients with IIH with tonsillar ectopia of ≥5 mm versus Chiari I would be needed to clarify the meaning of this finding.
Limitations and Future Directions
In the current study, patients with IIH were compared with healthy controls to determine the incidence and morphology of cerebellar tonsillar ectopia in IIH. Control cases were randomly selected from patients with normal MRI findings scanned for other reasons, rather than healthy volunteers.23 The use of control subjects with an average tonsillar ectopia of 0.7 ± 1.9 mm (range, −5 to 5 mm, 5/44 subjects having mild cerebellar tonsillar ectopia between 2 and 4 mm) may underestimate the difference in incidence of mild tonsillar ectopia between patients with IIH versus healthy controls because our control patients had a lower tonsillar position than previously reported for normal controls (as stated in the Discussion section). This secondary observation is intriguing because it may reflect geographic differences in the position of cerebellar tonsils relative to the FM, possibly reflecting a difference in average body mass index and preclinical IIH. However, a wide variation of normal is expected, and our sample size of healthy controls was small so that a conclusion regarding the variation of tonsil position among healthy controls cannot be made and was not the purpose of the current study.
A larger study of tonsillar position in healthy controls with close attention to body mass index, sex, and age would be needed to determine the validity and significance of this secondary observation. The measurement of the obex position in patients with IIH compared with controls showed a statistical difference, but no patients with Chiari I were included for comparison; therefore, the true meaning of this finding for distinguishing among the etiologies of cerebellar tonsillar ectopia is unclear. Future study of the obex position in patients with IIH and Chiari I would be helpful to determine the significance of this finding to distinguish among the different pathophysiologic etiologies of cerebellar tonsillar ectopia.
Conclusions
The cerebellar tonsillar position in patients with IIH is significantly lower than that in age-matched controls. A significant proportion (21%) of our patients with IIH had cerebellar tonsillar ectopia of ≥5 mm, which was often peglike, mimicking Chiari I. The obex position was significantly lower in patients with IIH, suggesting that the brain stem and cerebellum are both shifted inferiorly. When cerebellar tonsillar ectopia is identified, careful radiographic and clinical consideration of IIH is warranted to avoid misdiagnosis as Chiari I alone.
Footnotes
Paper previously presented at: 49th Annual Meeting of the American Society of Neuroradiology, June 4–9, 2011; Seattle, Washington.
Disclosures: Patricia Hudgins—UNRELATED: Consultancy, Royalties, and Stock/Stock Options: Amirsys, Comments: medical education company.
References
- Received December 1, 2011.
- Accepted after revision January 12, 2012.
- © 2012 by American Journal of Neuroradiology