Abstract
BACKGROUND AND PURPOSE: Localization of spinal CSF leaks in CSF hypovolemia is critical in directing focal therapy. In this retrospective review, our aim was to determine whether GdM was helpful in confirming and localizing spinal CSF leaks in patients in whom no leak was identified on a prior CTM.
MATERIALS AND METHODS: Forty-one symptomatic patients with clinical suspicion of SIH were referred for GdM after undergoing at least 1 CTM between February 2002 and August 2010. A retrospective review of the imaging and electronic medical records was performed on each patient.
RESULTS: In 17 of the 41 patients (41%), GdM was performed for follow-up of a previously documented leak at CTM. In the remaining 24 patients (59%), in whom GdM was performed for a suspected CSF leak, which was not identified on CTM, GdM localized the CSF leak in 5 of 24 patients (21%). In 1 of these 5 patients, GdM detected the site of leak despite negative findings on brain MR imaging, spine MR imaging, and CTM of the entire spine. Sixteen of 17 patients with previously identified leaks underwent interval treatment, and leaks were again identified in 12 of 17 (71%).
CONCLUSIONS: GdM is a useful technique in the highly select group of patients who have debilitating symptoms of SIH, a high clinical index of suspicion of spinal CSF leak, and no demonstrated leak on conventional CTM. Intrathecal injection of gadolinium contrast remains an off-label use and should be reserved for those patients who fail conventional CTM.
ABBREVIATIONS:
- CTM
- CT myelography
- GdM
- intrathecal gadolinium MR myelography
- In111-DTPA
- indium-111 diethylene triamine pentaacetic acid
- SIH
- spontaneous intracranial hypotension
- SPGR
- spoiled gradient-recalled-echo
SIH is a debilitating syndrome classically characterized by orthostatic headaches, low CSF pressure, and diffuse pachymeningeal gadolinium enhancement on MR imaging.1 First-line treatment for patients with this condition is conservative therapy or large-volume lumbar epidural blood patch.2 Further treatment, however, including targeted epidural blood patches, fibrin glue injections, and open surgical repairs may be necessary. Each of these focal therapies requires precise localization of the CSF leak. Current standard radiologic techniques used to evaluate spinal CSF leaks in these patients include conventional CTM, dynamic CT myelography, radionuclide cisternography, and conventional spine MR imaging. GdM has been reported in small series and case reports to be a useful adjunct in localization of CSF leaks in the difficult subset of patients with debilitating symptoms of SIH in whom no leak was identified on a prior CTM.3⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–15 However, the sensitivity of GdM in this subset of patients has not been well studied. Our aim in this study was to perform a retrospective review of our experience and to determine and describe the cases in which GdM was a helpful adjunct to CTM in localizing spinal CSF leaks.
Materials and Methods
Approval of the institutional review board with waived consent was obtained for this Health Insurance Portability and Accountability Act—compliant retrospective research study. A search of the radiology information system between February 2002 and August 2010 retrieved a total of 164 patients who were referred for myelography for evaluation of SIH. Of these, 41 patients underwent GdM after undergoing at least 1 CTM. If >1 GdM was performed on a single patient, only the first examination was evaluated in our study. No other cases were excluded.
Imaging examinations reviewed included prior brain MR imaging, spine MR imaging, CTM (standard or dynamic16), nuclear medicine In111-DTPA cisternography, and GdM. All imaging studies and reports were reviewed by a neuroradiologist (J.J.A.), and all GdM, by 2 neuroradiologists with consensus agreement (J.J.A. and P.H.L.). For each brain MR imaging, the presence or absence of diffuse pachymeningeal gadolinium enhancement, subdural fluid collections, and sagging of the brain was recorded. “Brain sagging” was defined as the subjective observation of inferior descent of the optic chiasm, midbrain, pons, or cerebellar tonsils with effacement of the suprasellar or prepontine cisterns on sagittal T1 images. Brain MR imaging was graded as “classic” if both pachymeningeal enhancement and sagging of the brain were present (Fig 1). Brain MR imaging was graded as “equivocal” if pachymeningeal enhancement without “brain sag” or “brain sag” without pachymeningeal enhancement was noted. Brain MR imaging was graded as negative if neither pachymeningeal enhancement nor sagging brain was present. For each spine MR imaging, the presence or absence of extradural fluid was recorded. For each CTM, the presence or absence of “CSF leak,” defined as an extradural collection of contrast unrelated to needle puncture, standard or dynamic technique, and delayed imaging time (if performed), was recorded.
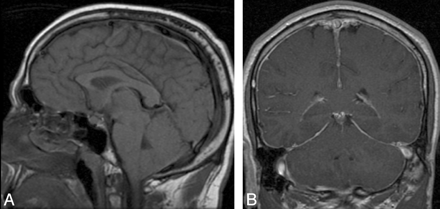
Patient with “classic” MR imaging findings of SIH on brain MR imaging. A, Brain sag on precontrast sagittal T1 imaging, with effacement of the suprasellar and prepontine cisterns, descent of the optic chiasm, draping of the floor of the third ventricle over the dorsum sella, descent of the midbrain, and extension of the tonsils through the foramen magnum. B, Diffuse pachymeningeal enhancement on postcontrast coronal T1 imaging.
For each GdM, the presence or absence of “CSF leak,” defined as an extradural collection of contrast unrelated to needle puncture was recorded, as well as the location of the leak. Successful localization was defined as localization of the leak to no more than 2 adjacent vertebral segments. For each leak, the predominant location of extradural contrast was recorded as foraminal, intraspinal extradural, or intralaminar. Additional GdM data recorded included assessment for adequate intrathecal opacification throughout the entire spine, assessment for artifacts at the cervicothoracic junction and at the posterior spinal tissues at C1/C2 related to poor fat saturation, the presence or absence of precontrast T1 fat-saturated imaging, and the presence or absence of delayed imaging.
Additional data obtained from chart review included patient demographics, therapy before GdM, therapy after GdM, any evidence of complications possibly related to intrathecal gadolinium administration (change in the character of headache, seizure, photophobia, or symptoms of arachnoiditis), and the most recent clinical note with the current status of the patient.
Our standard CTM protocol is as follows: After informed consent is obtained, a fluoroscopic-guided lumbar puncture is performed by using a 22-ga spinal needle. Intrathecal localization is confirmed, and approximately 16 mL of iohexol 180 (Omnipaque 180; GE Healthcare, Milwaukee, Wisconsin) is injected by using fluoroscopic visualization. Routine spot images are taken of the lumbar, thoracic, and cervical spine while the patient is placed in the Trendelenburg position. Care is taken to limit intracranial passage of contrast. The patient is then rolled several times to evenly distribute the contrast and is transferred immediately to the CT imaging suite where our routine CT protocol is performed. Currently, all CTMs are imaged on either a 64- or 128-section scanner with a minimum axial section thickness of ≤0.75 mm and additional reconstructions in the coronal and sagittal planes. These images are reviewed immediately after acquisition, and the patient is either dismissed or held for delayed imaging. Delayed imaging at 2–4 hours is often performed when no definite leak is identified on the initial imaging in patients with a high clinical suspicion of SIH.
Our standard GdM procedure is as follows: Informed consent is obtained with a discussion of the off-label use of gadolinium in the intrathecal space. Precontrast fat-saturated T1-weighted MR imaging of the cervical, thoracic, and lumbar spine in the sagittal plane is performed at the discretion of the radiologist. Fluoroscopic-guided lumbar puncture is performed by using a 22-ga spinal needle, and the subarachnoid location is confirmed by using 0.5–1.0 mL of iohexol 180 (Omnipaque 180, GE Healthcare). Following this confirmation, 0.5 mL of gadopentetate dimeglumine (Magnevist; Bayer Schering Pharma, Berlin, Germany), diluted in 5 mL of preservative-free 0.9% saline, is instilled into the thecal sac. At the discretion of the performing radiologist, the thecal sac pressure is then normalized by connecting a pressure monitor under sterile conditions to the intrathecal needle via a 3-way stopcock, and a volume of nonbacteriostatic sterile normal saline is slowly infused to raise the intrathecal pressure to 20–30 cm H2O. The needle is subsequently withdrawn. The contrast is distributed throughout the spinal canal by placing the patient in the Trendelenburg position. The patient is rolled several times to evenly distribute the contrast and then is immediately transferred to the MR imaging suite within 15 minutes of intrathecal gadolinium administration.
GdM is performed at 1.5T. Our routine GdM protocol includes fat-suppressed T1-weighted images of the cervical, thoracic, and lumbar spine in the sagittal (fast SPGR: 3.5 mm with 0.5-mm spacing; 15 sections centered on the spinal canal; TE autoadjusted by the scanner; TR, 230 ms; 256 × 192 matrix; 3 NEX; cervical FOV, 20 cm; thoracic and lumbar FOV, 28 cm), axial (fast SPGR: 4 mm with 0 spacing; TE autoadjusted by the scanner; TR, 160 ms; 256 × 192 matrix; 2 NEX; FOV, 20 cm), and coronal planes (fast SPGR: 4 mm with 0 spacing; 15 sections to cover from the spinous process through the vertebral body; TE autoadjusted by the scanner; TR, 230 ms; 512 × 192 matrix; 2 NEX; cervical FOV, 22 cm; thoracic and lumbar FOV, 28 cm). Delayed imaging after several hours with the same protocol is often performed, particularly if a definite leak is not seen on initial immediate imaging.
For both CTM and GdM, if delayed imaging is not performed, the patients remain supine for at least 1 hour and then are assessed by radiology nursing staff. If the patients report no symptoms, they are then discharged as outpatients with routine follow-up scheduled with their ordering physician.
Results
Population
Of a total of 164 patients who were referred for myelography for evaluation of SIH, 41 underwent GdM after undergoing at least 1 CTM. Before GdM, 41 of 41 of patients (100%) had a brain MR imaging, 36 of 41 (88%) had an MR imaging of the spine, and 28 of 41 (68%) had a nuclear medicine cisternography with In111-DTPA. Of the 41 patients (mean age, 51 years; range, 22–80 years), 14 (34%) were men and 27 (66%) were women.
General GdM Technical Results
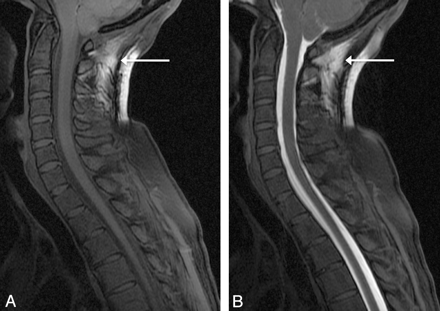
In 17 of our 41 patients, the thecal sac pressure was normalized with nonbacteriostatic sterile normal saline after instillation of gadolinium. Assessment for intrathecal opacification with gadolinium yielded only 1 of 41 patients (2%) who did not have sufficient intrathecal opacification of the cervical, thoracic, and lumbar spine, only sufficiently opacifying below the T3 level. Two patients did not have full opacification on the initial scan, but subsequently all levels were opacified on delayed imaging. Artifacts at C1-C2 were seen in 6 of 41 patients (15%) (Fig 2). However, no leaks were identified at the C1-C2 level, which has been reported by others as false localizing due to artifacts.17 Artifacts at the cervicothoracic junction due to poor fat saturation were seen in all 41 patients (100%).18 Precontrast T1 fat-saturated imaging was used in 34 of 41 patients to aid in the differentiation of artifacts and true leaks. The 7 patients who did not have precontrast T1 fat-saturated imaging did not demonstrate any focal leak at the cervicothoracic junction. Two of these 7 patients did have additional leaks found elsewhere.
A, Pre- and postintrathecal gadolinium fat-suppressed T1 images demonstrate typical artifacts, which may simulate a leak at C1-C2 seen in 6 of 41 of our patients. Note the inhomogeneous fat saturation on this precontrast sagittal T1 image at C1-2 (arrow). B, Recognizing this artifact on precontrast imaging is important because with the addition of intrathecal gadolinium, this inhomogeneous fat saturation can potentially mimic a leak (arrow).
Target Population for This Study
Our target population was those 24 patients referred for GdM in whom no leak was identified on prior CTM and in whom there were both debilitating symptoms and a strong clinical suspicion of SIH, which prompted referral for GdM. This group represented 14.6% (24 of 164) of our SIH referral population. Before GdM, all 24 patients (100%) had brain MR imaging and 21 of 24 (88%) had spine MR imaging. In addition, all 24 patients (100%) had a standard CTM, 5 of which were performed at outside institutions with variable techniques and 19 of which were performed at our institution. All of the 5 studies performed elsewhere were formally reviewed by our neuroradiology section before proceeding with GdM, and these reports were available for review. In 4 cases, the outside imaging was still available in our electronic archive. Four included the entire spine, and 2 included delayed images, 1 at 3 and 7 hours and 1 at 4 hours. The fifth study included the thoracic and lumbar spine without delayed images. A leak was subsequently identified at the L1-L2 level in this case.
Of the ones performed at our institution, 14 were 64-section CT with our standard protocol and a minimum axial section thickness of ≤0.75 mm, and 5 were performed before the arrival of 64-section CT with minimal section thickness ranging between 1 and 2.5 mm. Of the 19 CTMs performed at our institution, 16 (84%) had delayed imaging performed between 2 and 4 hours. Sixteen of 24 (67%) had a prior nuclear medicine cisternogram with In111-DTPA. Given the refractory nature of SIH, many of our patients underwent empiric treatment before GdM. Relevant therapies in our patient population include 20 of 24 (83%) who had prior epidural blood patch, and 4 of 24 (17%) who only had medical treatment, which typically included bed rest, oral hydration, oral pain medication, and generous caffeine intake. Three of 24 (13%) had spine surgery before referral for SIH. Two had craniocervical decompression surgery for “type 1 Chiari malformations,” which may have been misdiagnosed cases of SIH. One had a cervical laminectomy for pain. No leaks were identified at the surgical site in these 3 postoperative cases.
GdM in Patients with Negative CTM Findings (Target Population)
In 24 of the 41 patients in our study (59%), no leak could be detected on CTM, including on delayed CTM that was performed in 16 of these 24 patients (66%). A total of 5 (21%) leaks were found, 3 on initial GdM and 2 on delayed GdM, in these 24 patients. The leaks were successfully localized in 4 of these 5 patients. In 3 cases, the leaks were localized to the high thoracic region, including to the left T3 and T4 foramina, to the bilateral T1 and right T2 foramina, and to the right T1 and T2 foramina. In 1 case, the leak was identified extending dorsal to the thecal sac between the left L1 and L2 lamina, which was 3 levels above the level of needle placement. Targeted focal epidural blood patches were performed in 3 of these 4 cases with successfully localized leaks. Two patients with early follow-up at 1 week had a positive response to therapy. No follow-up was available in the third case. One patient with a localized leak had targeted epidural fibrin glue injection without improvement in symptoms. GdM was unable to localize the fifth leak, with initial GdM images demonstrating intraspinal extradural contrast from T11-L3 with contrast extending into multiple foramina bilaterally. Sequential epidural blood patches were performed at T11-T12, L2-L3, and L3-L4 without improvement in symptoms.
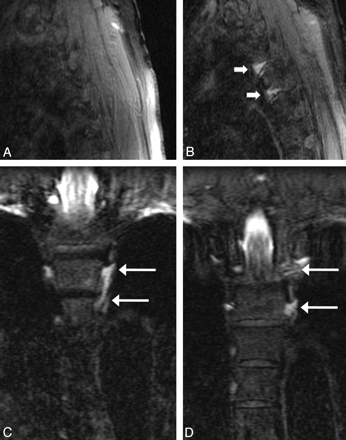
Overall, delayed GdM imaging was performed in 19 of the 24 patients (79%) in this subset at an average of 5.4 hours after the initial imaging (range, 0.75–24 hours). Of these 19, two patients (11%) demonstrated leaks only seen on the delayed images and 1 patient (5%) demonstrated progressive accumulation of contrast from a leak seen on the initial images (Fig 3). In the other 2 patients (11%) with GdM-documented leaks, the leaks were equally well seen on immediate and delayed imaging. Normalization of the CSF pressure was performed in 13 of the 24 (54%) patients, with 3 leaks discovered in this subset.
Patient with CSF leaks at the left T3–4 and T4–5 interspaces, which are only visible on delayed GdM. A, Immediate left parasagittal fat-suppressed T1 imaging with normal findings. B−D, Three-hour delayed left parasagittal fat-suppressed T1 imaging demonstrates the CSF leaks (arrows), a finding which is also documented in the coronal plane (C and D) with evidence of contrast within the T3 and T4 foramina (arrows).
Spine imaging was available in 21 of the 24 patients (86%) in this subset of patients with negative findings on CTM. Only 2 of 21 (9.5%) had extradural fluid. One of these 2 with extradural fluid and 4 of 19 (21%) without extradural fluid had leaks identified on GdM.
Of the 5 patients with GdM-documented leaks in this group, 2 patients had classic findings on brain MR imaging with negative spine MR imaging. One patient had equivocal findings on brain MR imaging with a negative spine MR imaging. The remaining 2 patients had negative findings on brain MR imaging, 1 of whom had an extradural fluid collection on the spine MR imaging. Four of the 5 patients had prior nuclear medicine cisternography. In 3 of the 4, the findings on cisternography were negative with no evidence of renal uptake at 4 hours. One patient had both positive findings on cisternography and a classic brain MR imaging. Thus, in 1 of these 5 patients with documented leaks, all prior imaging findings were negative.
GdM in Patients with Previously Identified Leaks on CTM
CTM had previously identified a leak in 17 of 41 patients. In 16 of these 17 patients, GdM was performed after intervening therapy, usually to avoid additional ionizing radiation. These were typically patients with complex conditions refractory to treatment with multiple prior CTMs and interventions, some of which had been performed elsewhere. GdM was of limited value in this patient population. While recurrent leak was confirmed in 12 of 17 (71%), in 7 of the 12 confirmed leaks, GdM was unable to localize the leak to within 2 adjacent vertebral segments due to the rapidity of the CSF leak and the poor time resolution of GdM. Spine imaging was available in 15 of the 17 patients with leaks identified on CTM. Extradural fluid was present in 6 of these 15 (40%).
Complications of GdM
One patient did have worsening of headache after GdM. This patient had no change in symptoms on discharge immediately after GdM. However, within 24 hours, this patient had increased headache, which was described as typical for the SIH symptoms. Placement of a lumbar epidural blood patch resulted in symptom resolution with no further complications. This increase in headache was thought likely to be a result of the lumbar puncture itself and not likely to be related to intrathecal gadolinium. No adverse gadolinium-related complications (seizure, photophobia, or symptoms of arachnoiditis) were observed in the remainder of the patients, with an average of 528 ± 643 days of follow-up (median, 311 days; range, 1–2490 days).
Discussion
During the past decade, SIH has been increasingly recognized as a cause of orthostatic headaches. In most cases, SIH is secondary to a spinal CSF leak.1,2 This has generated a significant interest in effective radiographic techniques to localize spinal CSF leaks. Beginning in the late 1990s, the use of low-dose intrathecal gadolinium began to be reported as an alternative to iodinated contrast for ventricular assessment,9,19 CSF rhinorrhea,4⇓–6,14 and spinal CSF leaks.3,15,20 These studies have illustrated the relative safety and utility of intrathecal gadolinium. Both Jinkins et al6 and Aydin et al5 have carefully assessed patients after low-dose intrathecal gadolinium administration with serial neurologic examinations. Aydin et al followed 51 patients with 1-, 3-, and 12-month neurologic evaluations and subsequent annual neurologic examinations for 3–6 years, with an average follow-up of 4 years, without evidence of any neurologic signs or symptoms that could be attributed to intrathecal gadolinium administration. Albayram et al3 and Yoo et al20 have demonstrated the utility of GdM for the assessment of spinal CSF leaks in consecutive series of patients with SIH in whom no other intrathecal contrast study had been performed to assess the leak.
We demonstrate that GdM plays a significant complementary role to CTM in the difficult subpopulation of patients with SIH who have debilitating symptoms and have not responded to conservative measures or blind large-volume lumbar blood patches and in whom carefully performed CTM typically with delayed images failed to demonstrate a spinal CSF leak. This group constitutes approximately 15% of all the patients referred to our myelography practice for SIH. In these, GdM detected leaks in 21%. While most presumed leaks in this problematic group of patients were still not detected, we think that the GdM studies added value to the diagnostic work-up and provided significant benefit, given the associated morbidity and mortality associated with intractable SIH.
An argument could be made that the added benefit we perceived is not from the increased sensitivity of GdM to slow CSF leaks but instead from repeat myelography, because repeat CTM has been shown in a few reported cases to document additional leaks, presumably because they are intermittent.21 While we cannot disprove this argument, we think that the relatively high percentage of leaks uncovered with GdM is difficult to explain only on the basis of intermittent leak. In addition, 3 of the 5 leaks were detected in the high thoracic region, where CTM is potentially degraded by artifacts from the shoulders. We think that CTM and GdM are complementary rather than duplicative.
In an effort to refine the indications for GdM in patients with prior negative findings on CTM, we attempted to correlate brain and spine MR imaging findings with the incidence of leak detected by GdM in this population (Table, groups 1–3). Although the population size was too small for statistical analysis, there was not a trend toward a higher incidence of leak in patients with positive brain MR imaging findings. However, caution must be applied because this observation may be due to this highly select population. Spine MR imaging has also been shown to be useful to assess extradural spinal CSF collections and structural causes of spinal CSF leak.2 The presence of an extradural fluid collection on MR imaging was seen in 2 patients, 1 of whom demonstrated a CSF leak on GdM. Normal brain and spine MR imaging findings should not preclude proceeding with GdM because we found a leak in 1 such patient with severe symptoms of SIH.
Categorization and distribution of patients with CSF leak identified on GdM
We demonstrate that delayed imaging at 4–6 hours is beneficial when the initial GdM is negative because 2 CSF leaks in our series were only seen on delayed imaging. Subtle CSF leaks noted on initial imaging can also be confirmed with progressive accumulation of contrast on delayed imaging, as seen in 1 additional case. In no cases were leaks more difficult to detect on delayed images. As a result, we recommend delayed imaging in all patients undergoing GdM who do not have an obvious leak on initial imaging.
Fat-saturation artifacts at the cervicothoracic junction and dorsal to the cervical lordosis can confound interpretation of GdM studies. For this reason, we have found obtaining pregadolinium fat-saturated T1 images to be of significant value.
Normalization of the intrathecal pressure was performed in 54% of our target population, and in 3 of the 6 leaks that were discovered. We postulated that CSF leaks are intermittent in some patients in whom CSF pressure decreases until the leak stops, with subsequent slow rise in pressure until the leak recurs. The rationale for normalizing CSF pressure is to avoid missing a CSF leak as a result of imaging at the nadir of this presumed cycle. However, we have neither sufficient data to demonstrate whether normalization of CSF pressure adds any additional diagnostic value nor data to determine whether it leads to false-positive results. This technique merits additional systematic study.
In a subgroup of the patients in this study, the GdM was performed to re-evaluate a previously documented CSF leak, mostly after interval therapy. Several authors4,9 have noted the relative benefit of avoiding ionizing radiation with GdM, and this was the motivation for using GdM in many of these patients. However, in our hands, GdM was not effective in localizing faster CSF leaks. Seven of the 12 leaks in this subgroup could not be localized because contrast extended in an extradural fluid collection over multiple spinal segments by the time the MR imaging was completed. The longer acquisition time of MR imaging compared with CT limits the ability of MR imaging to localize faster CSF leaks. In addition, GdM does expose the patient to the potential risk of intrathecal gadolinium. In patients with previously documented leaks who return with recurrent symptoms of SIH, limiting radiation dose by targeting CTM to the area of a previously identified leak may be more effective than using GdM to assess recurrence.
It has now been well-documented that intrathecal gadolinium at higher doses is neurotoxic.22 Despite the aforementioned clinical safety data and our successful use of GdM in selective cases during 8 years without known complications, this does not mean that the potential risk can be ignored. Offering both iodine-based and gadolinium-based studies in the same procedure room introduces the potential for a serious system error—inadvertent injection of the wrong contrast agent at a toxic dose. To help mitigate this potential safety risk in our practice, we no longer stock gadolinium-based contrast in our myelography procedure room. Rather, we obtain the contrast from our MR imaging suite immediately before a GdM study. In addition, all medication and contrast syringes should be clearly labeled at the time the syringes are loaded. We advocate frank discussion regarding the risk and benefits of GdM with patients, including a clear explanation that the intrathecal administration of gadolinium-based contrast is an off-label use. In addition, we do not recommend GdM as a first-line imaging choice for leak localization in SIH. Rather, we recommend an approach supported by our data: using GdM for those with previous negative findings on CTM in whom there is strong clinical suspicion of SIH.
Our study limitations include those inherent to a retrospective analysis, with a relatively small number of patients and single-center experience. All patients in whom CSF leak was diagnosed only by GdM underwent focal therapeutic intervention. However, none of these patients underwent open surgery with confirmation of a leak, and long-term follow-up was not available for all patients. In addition, the therapeutic response is known to be variable; this outcome limits the ability to use this response as the criterion standard to confirm the diagnosis of CSF leak. However, the tertiary referral base of problematic cases of SIH at our institution gives us the opportunity to define a limited role for GdM in a difficult patient population.
Conclusions
GdM is a useful technique in the highly select group of patients who have debilitating symptoms of SIH, a high clinical index of suspicion of spinal CSF leak, and no demonstrated leak on conventional CTM. Intrathecal injection of gadolinium contrast remains an off-label use but is thought to be an acceptable risk for this select patient population.
Footnotes
-
Paper previously presented at: 49th Annual Meeting of the American Society of Neuroradiology, June 4–9, 2011; Seattle, Washington.
References
- Received May 10, 2011.
- Accepted after revision June 25, 2011.
- © 2012 by American Journal of Neuroradiology