Abstract
BACKGROUND AND PURPOSE: A stable stroke experimental model is highly desirable for performing longitudinal studies using MR imaging. The purpose of this study is to establish a stable focal cerebral ischemia model with a high survival rate in adult mice.
MATERIALS AND METHODS: One hundred twenty adult mice were randomly divided into 10 groups of 12 each to respectively undergo intraluminal suture occlusion, with suture insertion depths from 0.8 cm to maximum; thromboembolic occlusion; and hypoxic-ischemic injury with hypoxia exposure times from 30–120 minutes. Coronal brain T2-weighted images were obtained on a 7T scanner. The induced infarct volume and location were assessed and correlated with histologic TTC staining. One-day and 7-day survival rates were recorded.
RESULTS: The infarct location was highly variable in the thromboembolic model, while it showed a cortex predominance in the intraluminal model with the suture insertion depth ≥1.4 cm, and the HI model with hypoxia exposure times ≥60 minutes (P = .001). The infarct volume in the intraluminal model with suture depths ≥1.4 cm (29.7 ± 3.3%, 35.4 ± 4.3%) and the HI model with the hypoxia exposure times ≥90 minutes (26.3 ± 4.1%, 33.4 ± 2.8%) were larger than other groups (9.7 ± 3.3%–20.9 ± 9.3%; P < .05). The HI group (72.5%) had higher 7-day survival rate than the intraluminal suture occlusion (28%) and thromboembolic occlusion groups (20%; P = .001).
CONCLUSIONS: The HI injury model with a reproducible ishemia and high survival rate can be used for a longitudinal study of brain ischemia in adult mice.
ABBREVIATIONS:
- CCA
- common carotid artery
- ECA
- external carotid artery
- HI
- hypoxia-ischemia
- MCAO
- middle cerebral artery occlusion
- PBS
- phosphate buffered saline
- PPA
- pterygopalatine artery
- TTC
- 2, 3, 5-triphenyltetrazolium chloride staining
Various focal cerebral ischemia experimental models simulating human stroke serve as an indispensable tool in the cerebral ischemia research field. Those models not requiring craniotomy are highly preferred, including intraluminal suture occlusion,1 thromboembolic occlusion,2 microembolization occlusion,3 photochemical thrombosis occlusion,4 and HI injury,5 and have been extensively used in rats.6 The mouse is the most appropriate animal on which to apply genetic modifications—it has been largely used in transgenic technology in studies of the molecular pathophysiology of stroke.7 However, most mouse focal cerebral ischemia models use an intraluminal suture or thromboembolic occlusion. Despite advancement of the procedures, these 2 methods are still associated with several disadvantages, such as high mortality rate and inconsistent infarct volume.7,8 Lesion reproducibility and size seem to be affected by many specific factors in intraluminal suture occlusion, such as suture diameter, coating of the suture, or insertion length of the thread. The infarcts induced by thromboembolic method are also highly variable in terms of location and size.7
Several sophisticated MR imaging modalities (eg, functional MR imaging, pharmacologic MR imaging, diffusion tensor imaging, molecular imaging) seem promising as novel tools for investigating many aspects of stroke.9⇓–11 The shortcomings of the intraluminal suture model and the thromboembolic occlusion model, that is, high variable cerebral lesion and high mortality rate, would hinder performing a longitudinal MR study to reveal the temporal evolution of ischemic lesion posttreatment on the same animals. Development of a stable focal cerebral ishemia model with high survival rate, where animals can be studied in a longer timeframe, is highly desirable for such in vivo longitudinal stroke studies.
The purpose of this study was to establish a stable model of focal ischemic cerebral injury with a high survival rate in adult mice, by observing the infarct location and size revealed on 7T MR imaging, as well as the survival rate between the HI injury model and other 2 typical models—the intraluminal suture occlusion model and the thromboembolic occlusion model.
Materials and Methods
Animals
All interventions and animal care procedures were performed in accordance with the Laboratory Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Guidelines and Policies for Animal Surgery provided by our university, and were approved by the institutional Animal Use and Care Committee. One hundred twenty adult male C57Bl/6J mice, 6 weeks old and between 20–25 g, were obtained from the Comparative Medicine Center of Yangzhou University, Yangzhou, China. All animals were housed in a standard animal facility with a 12-hr light-dark cycle and allowed standard food and water ad libitum.
One hundred twenty animals were randomly assigned to 10 groups of 12 each. The first 5 groups (groups I–V) underwent the intraluminal suture occlusion, with suture insertion depths of 0.8 cm–0.9 cm, 1.0 cm, 1.2 cm, 1.4 cm, and maximum (no longer advanceable), respectively. The sixth group (group VI) underwent thromboembolic occlusion. The last 4 groups (groups VII–X) received HI injury with hypoxia exposure times of 30, 60, 90, and 120 minutes, respectively.
Intraluminal Suture Model
The intraluminal suture model was produced according to the previously reported method.8 Briefly, after anesthesia with peritoneal injection of 1% pentobarbital sodium (6 mL/Kg), a middle cervical incision was made and the right submandibular gland was separated and the carotid sheath was exposed by a blunt splitting technique. The right CCA was carefully dissected from its bifurcation to the skull base. The thyroid artery and occipital artery branches of the ECA and PPA were ligated with a 5–0 nylon suture. The ECA was dissected and ligated proximally. Microvascular clips were placed on the ICA and CCA to block blood flow. A nylon poly-l-lysine-coated 2-cm suture with a blunt head (Model 1418; Sunbio Biotech, Beijing, China) was inserted via the proximal ECA into the ICA. The insertion lengths of the sutures were 0.8–0.9 cm, 1.0 cm, 1.2 cm, 1.4 cm, and maximum (until no longer advanceable). After 20 minutes occlusion, the clips were removed and the suture was withdrawn to restore blood supply to the MCA territory. The wounds were closed in layers and the animals were returned and housed separately.
Thromboembolic Model
The thromboembolic occlusion was established as described.12 In brief, fresh heterologous arterial blood (0.2 mL) was rapidly mixed with 0.04 mL thrombin (1 mg/mL, Sigma, St. Louis, Missouri) in an EP tube (Eppendorf, Hamburg, Germany) and then immediately transferred to a 30-cm microcatheter (PE-0402, ID = 0.2 mm, OD = 0.38 mm; Anlai, Ningbo, China). The microcatheter was placed at ambient pressure for 2 hours at 37°C to allow clot formation, followed by 48 hours clot stabilization at 4°C. Five-cm microcatheters were cut, and the clots were rinsed out and immersed in saline for 4 hours at 4°C to remove the remaining red blood cells. The fibrin-rich segments were dedicatedly selected under microscopy inspection, cut into small cylindrical pieces of 1–2 mm in length, suspended in PBS containing 1 mg/mL serum albumin, and again transferred into individual microcatheters. After animals were anesthetized with pentobarbital sodium (6 mL/Kg), the right CCA was exposed and the root of ECA, thyroid and occipital arteries, and PPA were ligated. A microvascular clip was placed on the CCA. The emboli-contained microcatheter was inserted through the incision of the ECA and was fixed with a suture. The clot was injected over a period of 5 seconds, and then the microvascular clip was removed and the microcatheter was withdrawn.
HI Model
The HI model was produced according to the previously reported method,5 with minor modification. Briefly, after the mice were anesthetized with pentobarbital sodium (6 mL/Kg), the carotid sheath was exposed and the right CCA was isolated and ligated with a 5–0 nylon suture. After animals recovered from the anesthesia, they were transferred to a chamber ventilated with a hypoxic gas mixture (8% O2 + 92% N2) at 1 L/min airflow. After 30-, 60-, 90-, or 120-minute hypoxia exposure, animals were retrieved from the hypoxia chamber and housed separately in a standard animal facility.
Neurologic Impairment Scoring
Twenty-four hours after surgery, neurologic impairment was assessed as described.1 The neurologic findings were scored on a 5-point scale: a score of 0 indicated no neurologic deficit, a score of 1 (failure to extend left forepaw fully) indicated a mild focal neurologic deficit, a score of 2 (circling to the left) indicated a moderate focal neurologic deficit, and a score of 3 (falling to the left) indicated a severe focal deficit; mice with a score of 4 could not walk spontaneously and had a depressed level of consciousness. Scores between 1 and 4 were considered modeling success.
MR Imaging
After neurologic assessment, MR imaging was performed on a 7T animal scanner (PharmaScan, Bruker, Germany) using a 23-mm mouse brain coil. Anesthesia was induced by 1.5% halothane and maintained with 1% halothane in 70% N2O and 30% O2. Animals were positioned in a plastic holder with a stereotaxic headframe, with heart rate and respiratory monitoring. Coronal and axial brain images were obtained with a rapid acquisition with relaxation enhancement T2-weighted imaging acquired with the following parameters: TR = 5000 ms, TE = 60 ms, section thickness = 0.5 mm, section gap = 0 mm, FOV = 35 mm × 35 mm, matrix size = 256 × 256, flip angle = 180°, number of signal intensity acquisitions = 6.
Imaging Evaluation
On T2-weighted images, the infarct volume was measured and the infarct location was observed. For infarct volume measurement, the obtained coronal MR images in DICOM format were processed in ImageJ software (National Institutes of Health, Bethesda, Maryland). Appropriate contrast enhancement of the images was completed to maximize the signal intensity from the hyperintense cerebral infarct region. Manual planimetry was performed. For each section, the hyperintense cerebral infarct region and total brain area were manually outlined. Section hyperintense areas were then summated to generate infarct volume as a percentage of total brain volume, according to the following formula13: infarct percentage = infarct volume / total brain volume * 100% = (A1 + A2 + A3 +. An) t / (V1 + V2 + V3 +. Vn) t, where A represents the infarct area, V represents the total brain area on each section, and t is the section thickness.
Survival Rate
At 1 day and 7 days postsurgery, the number of living animals in each group was recorded and survival rates were calculated.
Histologic Evaluation
At 1 day after MR imaging, 2 successfully modeled animals in each group were randomly sacrificed by overdose of anesthesia for TTC. The whole brain was removed and preserved at −80°C for 20 minutes and then cut into 6 coronal sections of 2-mm thickness. All sections were incubated with 2% TTC in PBS for 30 minutes at room temperature, then fixed in 4% paraformaldehyde for 5–6 hours. Images of the sections were acquired using a stereomicroscope equipped with a digital camera system (SEZ 300; Shenzhen Finial Technology, Shenzhen, China). TTC staining was evaluated as described.14 The infarct location and size were observed.
Statistical Analysis
The ordinal data were presented as means ± SD. The infarct volume was compared using ANOVA, followed by a post hoc Student-Newman-Keuls test. The sample size (n = 12 in each group) was determined as previously described,15 and a statistical power of 0.90 could be achieved using the ANOVA test. The survival rates and infarct locations were compared using Fisher exact test. A P value < .05 was considered statistically significant. All statistical analyses were performed with SPSS 13.0 software (SPSS, Chicago, Illinois).
Results
Modeling Success and Neurologic Scoring
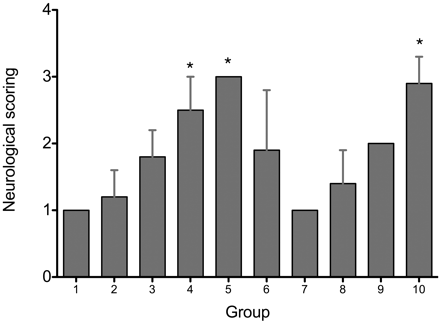
Successfully modeled animals are shown in Table 1. The overall success rate of the model was not statistically different between the intraluminal (90%, 54/60), thromboembolic (100%, 12/12), and HI (83%, 40/48) models (P = .34). The neurologic scoring is shown in Fig 1. The neurologic scoring was, respectively, 1.0 ± 0.0, 1.2 ± 0.4, 1.8 ± 0.4, 2.5 ± 0.5, 3.0 ± 0.0, 1.9 ± 0.9, 1.0 ± 0.0, 1.4 ± 0.5, 2.0 ± 0.0, and 2.9 ± 0.4 in groups I–X. Animals in groups IV, V, and X had higher neurologic scores than other groups (P < .05).
Modeling success and animal survival at 1 and 7 days after surgery
Graph shows the neurologic scoring in the intraluminal suture occlusion and thromboembolic occlusion and hypoxia-ischemia injury groups. * = P < .05 compared with other groups.
Infarct Size and Location
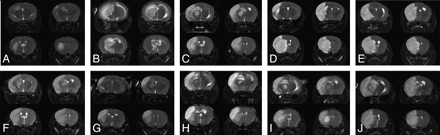
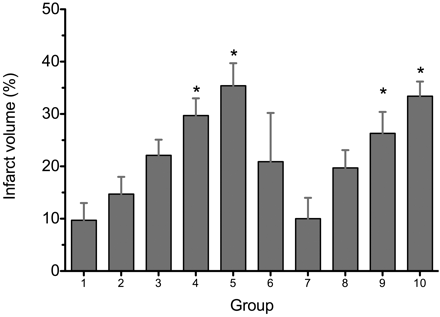
The infarct lesion showed hyperintense signal intensity on T2-weighted images (Fig 2). The lesion locations are shown in Table 2, and the measured infarct volume percentages are shown in Fig 3. The measured infarct volume percentage was, respectively, 9.7 ± 3.3%, 14.7 ± 3.3%, 22.1 ± 3.0%, 29.7 ± 3.3%, 35.4 ± 4.3%, 20.9 ± 9.3%, 10.0 ± 4.0%, 19.7 ± 3.4%, 26.3 ± 4.1%, and 33.4 ± 2.8% in groups I–X. The coefficient of variation of infarct volume was, respectively, 0.34, 0.22, 0.14, 0.11, 0.12, 0.44, 0.40, 0.17, 0.16, and 0.08, with the lowest found in group X and the highest in group VI. Animals in groups IV, V, IX, and X showed larger infarct volumes than other groups (P < .05). The infarct location in groups I–III in the intraluminal suture and thromboembolic models, and group VII of the HI model, was highly variable, while the infarct lesion in groups IV–V of the intraluminal suture model, and groups VIII–X of the HI model, had a cortex predominance (P = .001).
Coronal T2-weighted images show the lesion location and size in the intraluminal suture occlusion (A–E: groups I–V, thromboembolic occlusion (F: group VI), and hypoxia-ischemia injury groups (G–J: groups VII–X). The infract lesions show hyperintense signal intensity.
Graph shows the measured infarct volume percentage in the intraluminal suture occlusion, thromboembolic occlusion, and HI injury groups. * = P < .05 compared with other groups.
The infarct location observed on MR imaging
Survival Rate
The survival at 1 day and 7 days after surgery is shown in Table 1. One day after surgery, there were 43, 9, and 43 living animals, respectively, in the intraluminal suture occlusion, thromboembolic occlusion, and HI groups. The survival rate did not differ statistically among the 3 groups (P = .065). By 7 days after surgery, the number of living animals (excluding 2 animals sacrificed for TTC staining) was decreased, respectively, to 14, 2, and 29. The overall 7-day survival rate in the hypoxia-ischemia group (72.5%, 29/40) was significantly higher than that in the intraluminal suture occlusion (28%, 14/50) and thromboembolic occlusion (20%, 2/10) groups (P = .001). The 7-day survival rates in groups III, IV, V, VI, and X were relatively low.
TTC Staining
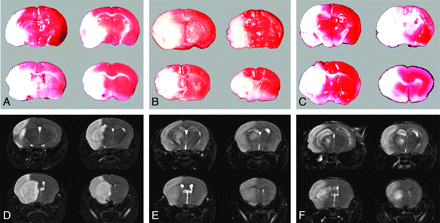
The infarct location and size detected by TTC staining were well matched with the MR imaging findings (Fig 4).
Brain TTC staining and the corresponding T2-weighted images in groups IV (A, D), VI (B, E), and IX (C, F) show that the infarct location and size are well matched.
Discussion
The results of our study suggest that intraluminal suture occlusion, thromboembolic occlusion, and HI modeling can achieve similar success. With the increase of the suture insertion depth or hypoxia exposure time, animals had higher neurologic scores and larger infarct volumes. The infarct location was highly variable in the thromboembolic model, while it showed a cortex predominance in the intraluminal model when the suture insertion depth was ≥1.4 cm, or in the HI model when the hypoxia exposure time was ≥60 minutes. The 7-day survival rate was highest in HI model compared with the intraluminal suture and thromboembolic occlusion models.
An experimental stroke model could be used to address specific questions either about pathologic events occurring after ischemic stroke or how to develop novel stroke therapies. The mouse is the most appropriate animal on which to apply genetic modifications,7 and transgenetic mice are now increasingly used to elucidate complex sequences of molecular events involved in ischemic injury.16 Intraluminal thread occlusion of the MCA (intraluminal occlusion model) and thromboembolic occlusion of the MCA either by infusing clots into the cerebral circulation (thromboembolic model)17 or by photochemically inducing thrombus16 are now 2 principal procedures used for mouse MCAO models. The intraluminal suture MCAO is the most frequently used model.1 This model provides reproducible MCA territory infarctions and allows reperfusion by retracting the suture.18 However, many factors—such as suture diameter (3.0 or 4.0 filament) and the application of a microvascular clip to the carotid artery,19 coating of the suture (with silicone or poly-L-lysine),20 occlusion duration,21 and even pterygopalatine arterial blood blocking8—affect the reproducibility of the infarct. With increase of the suture insertion depth, the procedure might easily result in subarachnoid hemorrhage or intracerebral hemorrhage.19 In our study, although a poly-l-lysine-coated suture (0.14 mm in diameter) was used and pterygopalatine arterial blood was blocked, the cerebral infarct size was indeed affected by the insertion length of the suture. Comparatively, the infarct volume as shown by MR imaging was high (29.7–35.4%) and infarct location seems constant (cortex predominance) when the suture insertion depth in the intraluminal model was more than 1.4 cm, but the 7-day survival rate was the lowest (0%). Such a low survival rate is unfavorable for performing a longitudinal stroke study on the same animal.
Thromboembolic stroke models mimic human stroke more closely than other models, as most human strokes are caused by thromboembolism. However, the ischemic lesions induced are highly variable in terms of location and size, and early spontaneous recanalization takes place.7 Our results suggest that the infarct location and volume in the thromboembolic model were highly variable and the 7-day survival rate was low (20%). This indicated that the thromboembolic model was also undesirable for a longitudinal study. Other than clots, photothrombosis could induce a cortical infarct through irradiation with a light beam at a specific wavelength after systemic injection of a photoactive dye.22 Recently, a mouse model of repeated thromboembolic stroke was established using photochemically induced CCA thrombosis followed by mechanical assistance. This procedure yielded a reliable and consistent brain infarct volume, with the lowest mortality at 3 days after surgery.16
Cerebral hypoxic-ischemic injury induced by unilateral ligation of the CCA, followed by a hypoxic exposure, could result in a stable cortical lesion and a high survival rate in neonatal rats.23,24 In our study, a hypoxic-ischemic injury was used on adult mice. Our results suggest that the lesion sizes were closely related to hypoxia exposure time. The infarct volume increased with the hypoxia exposure time. The infarct location demonstrated a cortex predominance when the hypoxia exposure time was more than 60 minutes. When the hypoxia exposure times were between 60 and 90 minutes, the 7-day survival rates achieved were 60–90%. Compared with the intraluminal and thromboembolic models, the overall 7-day survival rates in the HI model were the highest. These findings suggested that the HI model could facilitate a longitudinal investigation of cerebral ischemia injury on the same animals. Moreover, this procedure can be easily performed and is minimally invasive.
Our study had a limitation in that only conventional T2-weighted images were obtained to assess brain infarction. MR DWI and PWI are very sensitive to early ischemic changes. DWI is able to detect ischemic lesions in the hyperacute phase of ischemia, even within minutes of injury,25 and DWI signal intensity abnormalities correlate highly with the location and volume of pathologically confirmed cerebral infarction in animal stroke models.26 However, conventional T2-weighted imaging is readily available and it would be favorable for measuring infarct volume, because the infarct lesion and whole brain structure can be more clearly delineated and therefore easily defined on T2-weighted images.
Conclusions
The infarct location and volume in the intraluminal suture occlusion and thromboembolic occlusion models were highly variable and 7-day survival rates were low. The HI injury model can induce a stable and reproducible cerebral ischemic lesion in adult mice, with the highest 7-day survival rate. Although the underlying mechanism of HI injury is different from that of intraluminal or thromboembolic occlusion, it could be used for a longitudinal stroke study using adult mice in the field of restorative drug studies or neuronal repair evaluation.
Footnotes
-
This work is supported by National Natural Science Foundation of China (grant number 30870691) and Natural Science Foundation of Guangdong Province (grant number 9151008901000001)
Indicates open access to non-subscribers at www.ajnr.org
References
- Received May 6, 2011.
- Accepted after revision August 25, 2011.
- © 2012 by American Journal of Neuroradiology