Abstract
BACKGROUND AND PURPOSE: The location of the motor pathways in the PLIC remains controversial. In the current study, we trace the fibers from the tongue, face, hand, and foot motor cortices by using probabilistic diffusion tractography and define their somatotopic organization in the PLIC.
MATERIALS AND METHODS: Twenty subjects were retrospectively studied. Fiber tracts were separately calculated between ROIs in the cerebral peduncle and in the 4 different motor regions in the precentral gyrus. Probabilistic connectivity maps were generated, and the voxel with the highest probability was designated as the position of the motor pathway. The PI and LI were defined as the relative anteroposterior and mediolateral locations of the motor pathways.
RESULTS: Tongue pathways were located anteromedial to face in 16 hemispheres (40%), with P < .05 for the PI and LI. Face pathways were located anteromedial to hand in 25 hemispheres (62.5%) with P < .05 for PI and LI. Hand pathways were anteromedial to foot in 14 hemispheres (35%) and anterior in 11 hemispheres (27.5%), with P < .05 for PI but P > .13 for LI. Group analysis showed that the somatotopic arrangement of the bilateral hemispheres was symmetric.
CONCLUSIONS: Probabilistic tractography demonstrated the anteroposterior alignment of the motor pathways along the long axis in the PLIC. Probabilistic tractography successfully tracked the motor pathways of the tongue, face, hand, and foot from the precentral gyrus through their intersection with the larger superior longitudinal fasciculus to the PLIC in all cases, overcoming limitations of standard (nonprobabilistic) tractography methods.
ABBREVIATIONS:
- CBT
- corticobulbar tract
- conMap
- connecting fibers map
- CST
- corticospinal tract
- FACT
- fiber assignment by continuous tracking
- HARDI
- high angular resolution diffusion imaging
- LI
- lateral index
- M1
- primary motor tract
- PI
- posterior index
- PLIC
- posterior limb of the internal capsule
The motor pathways, including the CBT and the CST, are a group of white matter fibers carrying motor impulses that originate at the cortical level to the motor neurons in the brain stem and spinal cord. In addition to the academic interest of deepening our understanding of neuroanatomy, the exact anatomic localization of the motor pathways has a number of important clinical applications because they play critical functions in voluntary motion. For example, knowledge of the exact location of the motor pathways would improve the neurosurgical planning in patients with brain tumor or in neurosurgical deep brain stimulation.1 Nevertheless, the anatomic details of the internal organization of tracts, including the location and relationship of the 4 major components of the motor pathways (foot, hand, face, and tongue) as they pass through the PLIC remain conjectural.2,3 Historically, the internal organization of the motor pathways in the PLIC was based on electrical stimulation studies that indicated that motor tracts of the face and tongue were in the anterior aspect of the PLIC; the arm and hand were toward the middle and the leg and foot were seen somewhat more posteriorly.4 Recent studies based on DTI have demonstrated a relatively unanimous result that the CST location is in the posterior portion of the PLIC.3,5,6 However, the relationship between the various subdivisions of the CST in the PLIC fibers remains controversial.3,5⇓⇓⇓–9 Furthermore, the CBT was not described in these studies; this feature makes the depiction of the motor tracts incomplete.
FACT, an algorithm to produce specific tracts from diffusion tensor data, has been used successfully to define motor fibers for the hand and foot, including in the previously mentioned studies. However, understanding the relationship of the 4 major components of the motor tracts has been hampered because the FACT algorithm is limited in tracing the face and tongue tracts from the cortex through the PLIC. With the FACT method, the linear propagation approach converts the discrete voxel vector orientation into a continuous tracking line.10 By its nature, the FACT approach can only produce 1 reconstructed trajectory from a seed point. Hence, when a smaller white matter tract crosses a larger one, the tracking program will usually follow the larger crossing tract as opposed to the smaller tract that it was originally tracing.11,12 This characteristic creates a problem in attempts to trace the CBT of the face and tongue because as these smaller tracts cross the larger superior longitudinal fasciculus, the FACT algorithm follows the larger superior longitudinal fasciculus rather than the anatomically correct continuation of the motor tract.
Probabilistic tractography is a tracking method to obtain a connectivity index along a white matter pathway that reflects fiber organization.12⇓–14 This approach estimates a spatial distribution of a streamline arising from a single seed rather a single tract and is, therefore, capable of resolving fiber branching configurations. Pathway tracing can continue even if the probability is low for any single direction, which provides a more robust description of small or complex fiber architecture.14 To our knowledge, probabilistic tractography has not been previously applied to the question of the organization of motor pathway of the hand, foot, face, and tongue. In this study, we attempt to map the controversial location of these motor pathways in the PLIC by using probabilistic diffusion tractography. We hypothesize the following: 1) The motor fiber tracts originating from hand, foot, face, and tongue will be successfully mapped in the PLIC by using probabilistic tractography, and 2) the somatotopic organization of the tongue, face, hand, and foot tracts will proceed in an anteroposterior and mediolateral alignment along the long axis of the PLIC.
Materials and Methods
Subjects
This retrospective study was granted a Waiver of Authorization from the Institutional Review Board in full compliance with Health Insurance Portability and Accountability Act regulations. Eighteen patients with systemic cancers (breast cancer in 7, lymphoma in 2, and other in 9) and 2 healthy subjects underwent brain MR imaging with DTI. All subjects had normal results on the MR imaging after review by a board-certified radiologist holding a Certificate of Added Qualification in Neuroradiology and normal results on neurologic examination by a neurologist or oncologist. There were 13 females and 7 males, with a median age of 39.7 years (range, 17–54 years).
MR Imaging Sequences
MR imaging data were acquired with a 3T (Signa; GE Healthcare, Milwaukee, Wisconsin) scanner by using an 8-channel head coil. On the basis of localizer images, T1-weighted (TR = 600 ms, TE = 8 ms, thickness = 4.5 mm) and T2-weighted (TR = 4000 ms, TE = 102 ms, thickness = 4.5 mm) spin-echo axial sections, covering the whole brain, were obtained. 3D T1-weighted images (TR = 22 ms, TE = 4 ms, matrix = 256 × 256, thickness = 1.5 mm) were also acquired with a spoiled gradient-recalled acquisition in the steady-state sequence. DTI data were obtained with a spin-echo echo-planar imaging sequence (TR = 13,000 ms, TE = 89 ms, matrix = 128 × 128, voxel size = 1.88 × 1.88 × 3.00 mm, gradient orientations = 15, b-value = 1000 s/mm2).
Probabilistic Diffusion Tractography
DTI and FiberTools Software Package (Department of Diagnostic Radiology, University Hospital, Freiburg, Germany) implemented in Matlab (MathWorks, Natick, Massachusetts) was used to analyze DTI data.15 An ROI placed in the cerebral peduncle and ROIs placed in the tongue, face, hand, and foot motor homunculi in the precentral gyrus in both hemispheres were defined as seed regions. The probabilistic map from each seed region was calculated respectively. The conMap was chosen to illustrate the fiber tract between the cerebral peduncle and each motor area. This combines random walks from extended visiting maps arising from the 2 seed points to determine the uncertainty of the connection of a voxel to the seed points and to estimate the relative fractions of connecting fibers (directed toward the other seed area) versus merging fibers (directed toward a third nonseed area). Parameters were set to the following: number of random walks = 100,000, the maximum fiber length = 150 voxel, trace <0.002, fractional anisotropy >0.15.
ROIs
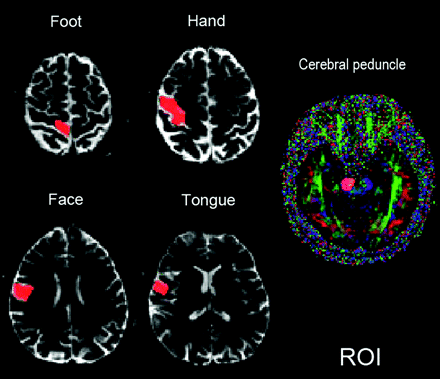
The motor pathways were traced for both hemispheres. First, an ROI was placed in the anterior part of the cerebral peduncle that was encoded in blue (indicating the craniocaudad direction of descending motor tracts) on the DTI color map. The other 4 cortical ROIs were placed over the tongue, face, hand, and foot motor areas in the precentral gyrus, a technique that has been previously validated16 (Fig 1). The foot ROI was placed in the uppermost portion of precentral gyrus and close to the midline of brain. The hand ROI was placed by identifying the “hand knob” of the primary motor cortex in the precentral gyrus.17 The face ROI was located in the lower portion of precentral gyrus and in the section of the top of the lateral ventricles. The tongue ROI was located in the lowermost portion of precentral gyrus and in the section just above the Sylvian fissure.18 All of the ROIs were determined by 2 board-certified neuroradiologists according to the anatomic landmarks displayed on the B0 images (the cerebral peduncle ROI in the DTI color map) in the axial plane.
An example shows the locations of the ROIs in the foot, hand, face, and tongue motor areas in the precentral gyrus of the right hemisphere. Motor ROIs are drawn by using B0 images in the axial plane. The ROI in the cerebral peduncle is drawn by using the color fractional anisotropy map.
Location of Motor Pathways in the PLIC
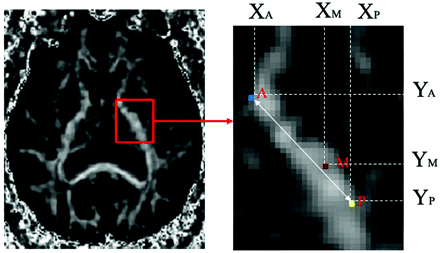
The motor pathways were localized in the PLIC at the level of the midthalamus. The anterior margin of the PLIC was defined as the medial apex of the lenticular nucleus, and the posterior margin of PLIC was defined as the posterior apex of the lenticular nucleus. The voxel showing the highest probability M (Xm, Ym) in each conMap between the cerebral peduncle and the motor area was defined as the position where the motor pathway traverses the PLIC. The position (X, Y) and probability value of every voxel in the probability map were measured. We defined point A (Xa, Ya) as the most anterior point of the long axis of PLIC and point P (Xp, Yp) as the most posterior point of the long axis of PLIC (Fig 2). To facilitate comparison, the relative locations of the motor pathways in the PLIC were measured by using anatomic landmarks for individual cases. The PI was defined as the relative anteroposterior localization of the motor pathway in the PLIC and was measured by using the following equation: PI = (Ym − Ya)/(Yp − Ya). The LI was defined as the relative mediaolateral localization of the motor pathway in the PLIC and was measured by using the following equation: LI = (Xm − Xa)/(Xp − Xa).
Determining coordinates in the PLIC. Point A is the most anterior point of the long axis of the PLIC. Point P is the posterior point of the long axis of PLIC, where the PLIC intersects the external capsule. In the above example, point M, representing the location, shows the highest probability value of a motor pathway in the PLIC.
Statistical Analysis
Paired Student t tests were performed to compare the mean difference of PI and LI between the tongue and face, tongue and hand, tongue and foot, face and hand, face and foot, and hand and foot. Results were considered significant at P < .05.
Results
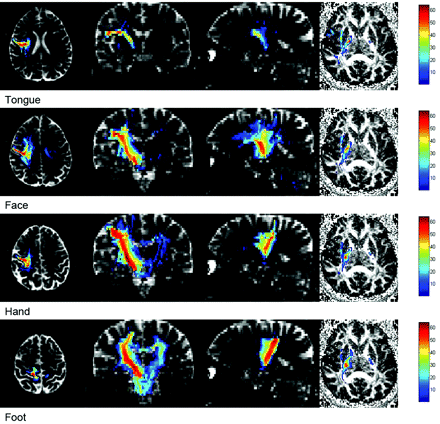
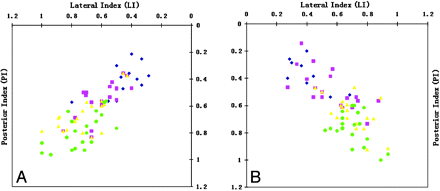
Using the probabilistic tracking method, we successfully mapped the 4 motor subpathways (tongue, face, hand, and foot) bilaterally in all 20 subjects, for a total of 160 tracts. A representative example showing all of the motor pathways traversing the PLIC is shown in Fig 3. The posterior and lateral indexes of the motor pathway in the PLIC for each subject were plotted for both hemispheres (Fig 4). Statistical comparison between motor pathways is displayed in Table 1.
Probabilistic connectivity pathway for a single subject overlaid on the multiplane B0 image (axial, coronal, and sagittal) and fractional anisotropy map (axial image at the level of PLIC). It shows the motor pathways originating from the tongue, face, hand, and foot in precentral gyrus extending to the cerebral peduncle. The connectivity map, overlaid on the fractional anisotropy map, shows that the pathways traverse the different parts of PLIC. The red areas indicate the highest probabilistic connectivity.
Scatterplot shows the coordinates of the PI and the LI for each motor pathway for each subject. The motor pathways for the tongue (blue), face (pink), hand (yellow), and foot (green) are presented in an anteromedial-to-posterolateral distribution along the long axis of the PLIC. The somatotopic arrangements for both right (A) and left (B) are compared.
Pair-wise comparisons of PI and LI of motor pathways in PLIC in both hemispheresa
Tongue and Face
In 18 of the 40 tracts (45%), the tongue pathways were located in the same voxel as the face pathways in the PLIC. In 16 tracts (40%), the tongue pathways were located anteromedial to the face pathways. In the remaining 6 tracts, the tongue pathways with respect to face pathways were anterior in 1 (2.5%), lateral in 3 (7.5%), and medial in 2 (5%). The mean PI (right: P = .004; left: P = .026) and mean LI (right: P = .002; left: P = .027) of the tongue motor pathways were lower than those of the face motor pathways.
Tongue and Hand
In 33 tracts (82.5%), the tongue pathways were located anteromedial to the hand pathways. In 4 of the 40 tracts (10%), the tongue pathways were located in the same voxel as the hand pathways in the PLIC. In the remaining 3 tracts, the tongue pathways with respect to the face pathways were anterior in 1 (2.5%) and medial in 2 (5%). The mean bilateral PI (P < .001) and LI (P < .001) of the tongue motor pathways were lower than those of the hand motor pathways.
Tongue and Foot
In 39 tracts (97.5%), the tongue pathways were located anteromedial to the foot pathways. In the remaining tract, the tongue pathway was anterior to the foot pathways. The mean bilateral PI (P < .001) and LI (P < .001) of the tongue motor pathways were lower than those of the hand motor pathways.
Face and Hand
In 25 tracts (62.5%), the face pathways were located anteromedial to the hand pathways. In 9 of the 40 tracts (22.5%), the face pathways were located in the same voxel as the hand pathways. The face pathways to the hand pathways were anterior in 2 (5%), lateral in 1 (2.5%), medial in 1 (2.5%), and posterior in 2 (5%). The mean PI (right: P = .001; left: P < .001) and mean LI (P < .001) of the face motor pathways were lower than those of the hand motor pathways.
Face and Foot
In 35 tracts (87.5%), the face pathways were located anteromedial to the foot pathways. The face pathways with respect to foot pathways were anterior in 3 (7.5%) and posterior in 2 (5%). The mean bilateral PI (P < .001) and mean LI (P < .001) of the face motor pathways were lower than those of the hand motor pathways.
Hand and Foot
In 2 tracts (5%), the hand pathways were located in the same voxel as the foot pathways. In 14 tracts (35%), the hand pathways were located anteromedial to the foot pathways. In 11 tracts (27.5%), the hand pathways were located anterior to the foot pathways. The hand pathways to the foot pathways were anterolateral in 6 (15%), medial in 2 (5%), and posterior in 5 (12.5%). The mean PI (right: P < .001; left: P = .001) of the hand motor pathways was lower than that of the foot motor pathways, but the difference was not significant in for the LI (right: P = .127; left: P = .370) (Fig 4 and Table 1).
Left and Right
No differences were observed between the hemispheres in terms of the tongue, face, hand, and foot motor pathway in the PLIC (P > .05) (Table 2).
Comparison of average location of motor pathways according to the hemispheresa
Discussion
In the current study, we successfully traced the motor pathways of the tongue, face, hand, and foot in the PLIC by using probabilistic diffusion tractography. Our results demonstrated a somatotopic organization of the motor subtracts with an anteroposterior alignment along the long axis of the PLIC. As a rule, the tongue pathways were located anteromedial to the face, the face pathways were located anteromedial to the hand, and the hand pathways were located anterior to the foot with some interindividual variability. Group analysis results showed that the bilateral hemispheres somatotopic arrangement was symmetric.
We analyzed each axial component (x and y) independently because there are conflicting reports in the literature regarding the internal organization of the subdivisions of the CST. Two studies3,5 reported that hand fibers were located anterolateral to foot fibers along the short axis of the PLIC rather than along the long axis. Our results are in a good agreement with a previous study conducted during brain surgery4 and a more recent study conducted by using diffusion tractography,9 which showed that the motor pathways were organized along the long axis of the PLIC. A recent study by using DTI in 27 patients with capsular and pericapsular acute infarctions showed that the motor weakness of face, upper extremities, and lower extremities was found mostly involving the anterior, middle, and posterior parts of the CST, respectively.19 The reason for the discrepancy is not immediately clear. However, the results of the studies, which implied organization along the short axis of the PLIC, used FACT-based tractography of the CST and were limited by small sample sizes.
The motor homunculus at the level of the motor strip cortex is known to lie in a horizontally oriented position.20⇓–22 The foot part of the homunculus is located at the most medial part of the precentral gyrus, and the hand part is lateral to the foot, whereas the tongue and face part are located at the most lateral aspect. Recently, several studies9,23⇓–25 reported the location of the CST as an anterolateral-to-posteromedial alignment in the corona radiata and a medial-to-lateral alignment at the midbrain level. Other investigators8,22 proposed that the foot fibers form an axis of rotation, around which the CST rotates anteriorly approximate 90° as they descended from the precentral gyrus to the internal capsule. According to their study, the motor homunculus should have an anteroposterior alignment along the long axis of the PLIC. The results of our study are in a good agreement with the results of Yamada et al,26 indicating that the hand pathways are located anterior to the foot pathways in the PLIC. Furthermore, our results demonstrate that the CBT (tongue and face fibers) is oriented anterior to the CST in the internal capsule. Given the linear relationships between the motor areas of foot, hand, face, and tongue in the motor strip, the organization of the motor pathways in the PLIC that we have proposed appears to make more anatomic sense than does any other model.
In the present study, probabilistic tractography was used successfully to determine the most probable motor pathways connecting the precentral gyrus and the cerebral peduncle. The relative probability of any single direction can be estimated and quantified by the algorithm in fiber-crossing regions. Therefore, the quantitative connectivity distribution allows one to define the most probable location of every single motor pathway in the PLIC. We showed that every subject's somatotopic organization of the motor tracts in the internal capsule was not completely consistent and some interindividual variability does exist. In each case, the organization of the motor tracts in the internal capsule demonstrated a topographic distribution along the long axis of the PLIC, though there are some overlaps. Group result comparison of the motor pathways confirmed that their relative locations are clearly aligned antero-posteriorly. Furthermore, the close SD of every single motor pathway (from ±0.103 to ±0.167) indicated that position points of every motor pathway were spread out over a similar range of values, which reflected the robustness and the reproducibility of the probabilistic method. Data of bilateral hemispheres suggested no significant difference between the average relative location of motor fibers in the left and right. Therefore, the somatotopic organization of motor pathways in the bilateral PLIC is symmetric. This finding enabled us to confirm that the group analysis of probabilistic data is a valid technique for depicting the anatomy of white matter bundles in the brain.
Determining the internal organization of the CST by using probabilistic methods of analysis of the diffusion tensor data has been previously performed by Zarei et al.12 However, these authors described the white matter trajectories of large subdivisions of the hemispheres (the prefrontal, premotor, M1, primary somatosensory, posterior parietal, temporal, and occipital cortices), whereas we defined the subdivisions of the projections of the motor cortex, including the CST and the CBT. In terms of technique, Zarei et al12 showed all tracts that were above a certain threshold, whereas we showed only the point of highest probability. Notwithstanding the differences in technique, both studies showed a large overlap in the white matter projections: between the subdivisions of the CST and the CBT in the present study and between the premotor cortex and M1 and the M1 and the sensory or thalamocortical tracts in Zarei et al.12
The HARDI technique is reported to be able to solve the problem of crossing fibers.26 However, long scanning and calculation times may hamper its use in clinical cases. The acquisition time of the whole brain by using the HARDI technique is usually >25 minutes.26,27 Given that our DTI data came from routine clinical scans (the scanning time for the diffusion sequence was <5 minutes), which allows one to start tracking the fibers soon after the completion of data acquisition, the present study should have more clinical implications for neurology and neurosurgery. The somatotopic organization of motor pathways should help neurologists explain the mechanism of clinical symptoms; for example, the absence of motor deficit occurs in patients with infarct, multiple sclerosis, or brain tumor involving the PLIC. Similarly, understanding the somatotopic organization of motor pathways should guide a neurosurgeon in planning the resection of a brain tumor located near the PLIC and will guide him or her to avoid important white matter fibers.
One potential limitation to the probabilistic approach in this study is the uncertainty of the technique, particularly for the lateral motor pathways such as face and tongue. These tracts have higher uncertainty due to smaller fiber bundle volume, sharper path inflection, and crossing fibers (ie, the superior longitudinal fasciculus) than the CSTs that have a much higher probabilistic connectivity. Our results support the contention that the probabilistic method is also more sensitive to major crossing pathways. Hence, quantitative comparison between different fiber pathways is difficult.28
Second, we found that 2 pathways, for example tongue and face or face and hand, were located in the same voxel in a number of cases. Rarely, the orientation was opposite to what was expected and what was seen in most cases. Therefore, one can conclude that by using the current methodology, it is occasionally difficult to determine whether the 2 pathways are mixed or very close. As discussed, the voxel with the highest probability in the probability map indicates the most probable location of the fiber bundle. How to determine the width and center of these fiber bundles, however, is not known and requires further investigation.29
The current study was also limited by only 15 gradient orientations, obtained during routine clinical scanning. Using an increased number of diffusion directions and a multitensor approach, one should be able to obtain more accurate and detailed information about diffusion in the brain, which may increase the sensitivity to complex fiber structures such as crossing fibers.26,30 Also, fMRI should be able to more precisely identify cortical activation areas in individual subjects than using anatomic landmarks because of its excellent spatial resolution at the cortex and high sensitivity to interindividual variability. Although beyond the scope of the current retrospective study, these strategies may be used in a future study.
Conclusions
Probabilistic tractography successfully tracked the motor pathways of the tongue, face, hand, and foot from the precentral gyrus through their intersection with the larger superior longitudinal fasciculus to the PLIC in all cases, overcoming limitations of standard (nonprobabilistic) tractography methods. The motor subtracts aligned along the long axis of the PLIC in an anteroposterior orientation. The tongue pathways were located anteromedial to the face; the face, anteromedial to the hand; and the hand variably, anterior to the foot. To the best of our knowledge, this is the first DTI tractography study to investigate the location of the CSTs and CBTs in the PLIC at the same time. The present results should have clinical implication for neurosurgery and neurology.
References
- Received July 21, 2011.
- Accepted after revision October 20, 2011.
- © 2012 by American Journal of Neuroradiology