Abstract
BACKGROUND AND PURPOSE: Carotid artery–related stroke is largely an embolic disease that has been correlated with inflammation, plaque rupture, and thrombus formation in “vulnerable” atherosclerotic plaque. Nevertheless, current guidelines for carotid revascularization in asymptomatic patients rely on the calculation of stenosis for risk assessment, a parameter that has been viewed with increasing skepticism. Intravascular OCT is an imaging technique that offers high axial resolution (10 μm), allowing an unprecedented micron-level assessment of human carotid plaque morphology. This observational article reports the first successful use of the newest iteration of this technology, FDOCT without balloon occlusion to assess human carotid artery disease and carotid stent-vessel interaction in vivo.
MATERIALS AND METHODS: Four patients with asymptomatic carotid artery disease and ambiguous noninvasive and/or angiographic data underwent carotid FDOCT to assess risk and to formulate a treatment strategy.
RESULTS: Findings include the unexpected demonstration of TCFAs, plaque rupture, thrombus, inflammation, and marked tissue prolapse through stent struts in patients without high-risk factors by conventional criteria, as well as low-risk features in a patient with a high-risk noninvasive study. The procedures were performed without safety issues or special accommodations for vessel occlusion.
CONCLUSIONS: The present study demonstrates the technical feasibility of FDOCT in cervical carotid arteries. As such, this technology holds the promise of not only clarifying ambiguous data in individual patients but of providing data that might call for a future paradigm shift in the assessment of asymptomatic carotid artery disease.
ABBREVIATIONS:
- CCA
- common carotid artery
- FDOCT
- frequency-domain optical coherence tomography
- NIH
- neointimal hyperplasia
- OCT
- optical coherence tomography
- TCFA
- thin-cap fibroatheroma
- TDOCT
- time-domain optical coherence tomography
Stroke is the fourth leading cause of death and a leading cause of long-term disability in the United States. Recent estimates list carotid artery atherosclerosis as a culprit in 15%–20% of these cases.1 Almost two-thirds of these strokes affect patients who were previously asymptomatic.2 The challenge to identify patients with asymptomatic carotid atherosclerosis who are at risk for stroke is daunting because of both the frequent occurrence of carotid atherosclerosis in the elderly3 and an increasing awareness that an estimation of the degree of stenosis in a carotid lesion is a poor predictor of clinical outcome.4 Numerous studies have suggested that stroke risk may be best predicted by plaque morphology in these asymptomatic lesions.5⇓–7 The value of MR imaging8 and multidetector CT imaging,4 as well as intravascular sonography9 to evaluate carotid disease has been reported but not validated in multicenter trials or accepted into general practice.
Intravascular OCT is an emerging technique recently approved for use in the United States. This imaging technique, analogous to intravascular sonography with the substitution of light for acoustic energy, delivers resolution to 10–20 μm, giving it the highest imaging resolution of any currently available intravascular imaging technique.10 Furthermore, the variable tissue penetration of light (ranging from 1 to 3.5 mm, depending on tissue composition),10 when combined with pixel intensity provides accurate identification of lipid-rich11 and calcified plaques12 and intraluminal thrombus.13 In addition, OCT makes it possible to directly visualize and quantify TCFA14 and vascular inflammation.15 These important features of vulnerable plaque, heretofore largely invisible by other imaging modalities due to their lower resolution, are readily identified and quantified with the micron-scale resolution of OCT. The latest iteration of OCT, FDOCT compared with TDOCT, moreover obviates the need for vessel occlusion by allowing an acquisition speed of up to 25 mm/s.
Detailed description of both systems is beyond the scope of this article. Briefly, while TDOCT uses a moving mirror as its reference arm, FDOCT uses a fixed mirror with a variable frequency light source, allowing simultaneous detection of reflections from all TE delays, making the system significantly faster. The larger (approximately 10 mm) FOV in FDOCT images also allows examinations of larger vessels, such as the carotid arteries.10
We report, for the first time, the successful use of FDOCT in vivo using a nonocclusive technique in the carotid arteries. OCT was used to clarify ambiguous noninvasive and angiographic carotid data in 3 cases with the resultant demonstration of interesting findings that suggest that markers for stroke risk heretofore only previously described by ex vivo histopathologic studies may be captured in vivo by means of OCT. In a fourth case, OCT ruled out features of plaque complexity previously suggested by a carotid duplex test.
Materials and Methods
Four patients undergoing diagnostic carotid angiography with standard 5F diagnostic catheters underwent ad hoc OCT assessment of ICA lesions. No patient came to the angiographic suite for a planned interventional procedure because it is our policy to perform diagnostic carotid and cerebral angiography on all patients before any recommendations for revascularization. Indications included the clarification of abnormal nonspecific carotid angiograms (patients A, C, and D) and the resolution of discordant angiographic-duplex findings (patient B). All patients were asymptomatic. Patient selection and immediate treatment was under the auspices of an interventional cardiologist with coronary OCT experience (M.R.J.), an interventional neuroradiologist (C.A.G.), and an endovascular-trained neurosurgeon (W.H.B.). All patients gave written informed consent for procedures performed.
Following angiography and before OCT interrogation, all patients underwent systemic anticoagulation with unfractionated heparin, achieving a target activated clotting time of >250 seconds. Three patients (A, C, and D) were studied with7F Arrow-Flex Sheaths (Arrow International, Reading, Pennsylvania). Patient B was studied using a 6F Shuttle sheath (Cook, Bloomington, Indiana). Embolic protection (NAV-6 Filterwire; Abbott Vascular, Redwood City, California) was used in patients A and C only.
In each case, the delivered guidewires were used to advance a 2.7F C7 Dragonfly catheter (St Jude Medical, St Paul, Minnesota) across the lesion. Data were then acquired with a C7-XR OCT Intravascular Imaging System (St Jude Medical) and digitally stored. All OCT automated pullbacks covered 54 mm of vessel at 20 mm/s. During image acquisition, carotid blood flow was replaced by hand injections of contrast using a 30-mL syringe. Contrast was chosen in lieu of saline because of the efficiency of these high-viscosity agents in displacing signal-intensity-attenuating red blood cells during the data-acquisition period.10 The average contrast use was 20 mL per acquisition run. Contrast delivery was achieved in <3 seconds per acquisition.
2D images were reconstructed on-line and used to facilitate clinical decision-making in the catheterization laboratory. Additional off-line imaging analysis and 3D reconstructions were conducted by 2 experienced OCT analysts blinded to patient characteristics at the Imaging Core Lab, University Hospitals, Case Medical Center, Cleveland, Ohio (H.G.B. and G.F.A.) by using proprietary software (St Jude Medical). All cross-sectional images (every 0.2 mm) were analyzed after an initial screening for quality and exclusion of any image from analysis in which a portion of the vessel was out of view or in which residual blood impaired analysis. We defined “thrombus” as any mass protruding into the lumen, with an irregular surface and a sharp intensity gap between the mass and surrounding tissue.16 We also performed semiautomated calcium segmentation17 and fibrous cap quantification in 1 of the cases with a custom-built software by using Matlab (MathWorks, Natick, Massachusetts). Cap thickness was quantified with respect to the centroid of the lumen and rendered in a color code display, as follows: pink (cap thickness < 65 μm), green (65–149 μm), and blue (150–220 μm).18
Results
Patient A
Patient A was a 64-year-old asymptomatic man with 80% angiographic stenosis of the left ICA by NASCET criteria. The patient was considered “high surgical risk” due to severe chronic obstructive airways disease and was enrolled in the Abbott-sponsored CHOICE carotid stent registry (http://www.strokecenter.org/trials/clinicalstudies/choice-carotid-stenting-for-high-surgical-risk-patients).
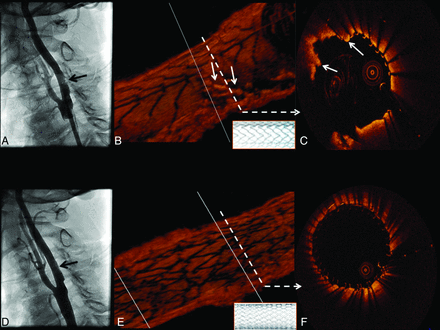
Following placement of a 10 × 40 mm open-cell RX Acculink carotid stent system (Abbott Vascular), deployed with a 6-mm Viatrac balloon (Abbott Vascular), a postdeployment angiogram demonstrated an unusual eccentric area of nonspecific “haziness” in the stented area (Fig 1). OCT was then performed with the demonstration of a large area of severe tissue prolapse without thrombus through the open cells in the Acculink stent (Fig 1B, -C). Prolapse was treated with the placement of a 10 × 30 mm closed-cell Xact carotid stent system (Abbott Vascular), with the resolution of prolapse by angiography (Fig 1D) and OCT (Fig 1E, -F).
Tissue prolapse after stent implantation: an angiographic and 3D OCT evaluation. Angiography shows an inconclusive “haziness” inside the proximal edge of the stent (A, black arrow). B, 3D view of the stented carotid artery in which tissue prolapse is depicted (solid white arrows). The open-cell stent design (inlay with white background) is clearly demonstrated by the 3D image. The cross-section represented in C also shows tissue prolapse through the stent struts (solid white arrows). A good final angiographic result after the placement of a closed-cell stent is shown in D (black arrow). OCT 3D longitudinal reconstruction also reveals a different stent design (inlay with white background) and no significant remaining tissue prolapse (E), which can be confirmed by cross-sectional assessment (F).
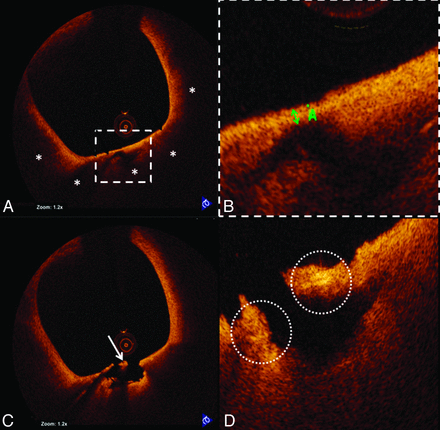
OCT also demonstrated a large TCFA with possible macrophage infiltration, plaque rupture, and white thrombus proximal to the stented segment (Fig 2). This segment was not obstructive and could not be detected by angiography. Hence, no additional intervention was performed.
TCFA rupture and inflammation visualized by OCT. A, Representative cross-sectional OCT image of a TCFA. The fibrous cap overlies a signal-intensity-poor region (white asterisks), corresponding to a lipid-rich plaque. B, The thickness (green double arrow) of the fibrous cap is 60 μm. A site of plaque rupture is shown in C, highlighting the presence of white thrombus (white arrow). D, The site of rupture also demonstrates bright spots that likely correspond to macrophage infiltration (dashed white circles) along the fibrous cap.
Patient B
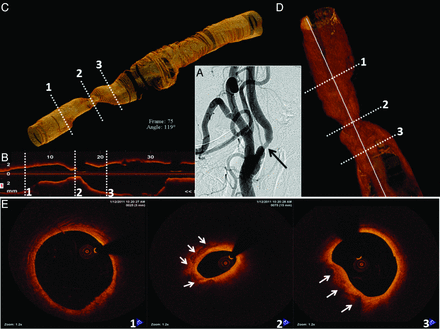
Patient B was a 70-year-old asymptomatic man with an abnormal right ICA duplex that demonstrated isoechoic and hyperechoic plaque with marked elevation of flow velocities (peak systolic = 419 cm/s; peak diastolic = 165 cm/s) predictive of high-degree ICA stenosis. The contralateral vessel was patent. Cerebral angiography revealed only a 60% right ICA stenosis by NASCET criteria (Fig 3 A).
OCT 3D reconstruction of the right ICA. A, Moderate stenosis in the ICA is also demonstrated in the OCT longitudinal view in B. C and D, 3D OCT reconstructions reveal the region of interest along with proximal and distal reference segments. The numbered white dashed lines correspond to the cross-sectional images in E. A normal distal reference vessel is depicted in 1. The minimal luminal area (2) reveals a calcified plaque (solid white arrows). Signs of plaque instability are absent. The proximal reference area (3) exhibits a patent lumen with the presence of calcium (solid white arrows).
OCT was performed to help determine the severity and complexity of disease, given the discordant findings between noninvasive and invasive studies. OCT revealed a plaque with 55% diameter stenosis at the site of minimal luminal area in the ICA (OCT-derived NASCET criteria). The plaque had areas of calcium (Fig 3) with no signs of plaque instability, such as a large lipid core, thin fibrous cap, erosion, rupture, or thrombus. Intervention was deferred on the basis of such findings.
Patient C
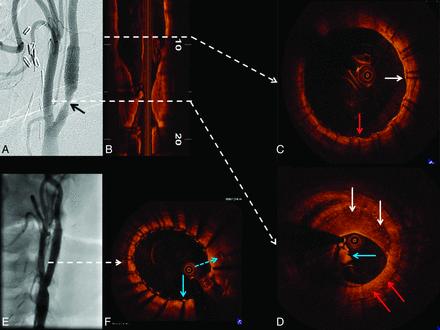
Patient C was a 60-year-old man who underwent a left carotid endarterectomy in 1997 and carotid stent placement for carotid restenosis (VIVA Registry: Vivexx stent, C.R. Bard, Murray Hill, New Jersey) in 2006. Carotid angiography performed to evaluate an abnormal ICA duplex study revealed an unusual 60% (NASCET) in-stent restenosis with an area of marked hypolucence in all views (Fig 4 A). OCT was undertaken to evaluate these abnormal angiographic findings in a patient with a history of both surgical and in-stent restenosis in the target vessel. Findings included a clearly delineated restenotic area with 65% diameter stenosis (OCT-derived NASCET criteria) composed of 2 different patterns of neointimal hyperplasia: a homogeneous signal-intensity-rich area, and a heterogeneous region with predominantly poor signal-intensity tissue. Moreover, a large area with irregular contour composed of white thrombus (Fig 4D), a marker for clinical risk by pathologic studies, was revealed. Despite the patient's asymptomatic status and with only 60% angiographic stenosis, intervention (stent placement with distal embolic protection) was performed (Fig 4E, -F).
OCT evaluation of ICA in-stent restenosis. Angiography reveals ICA in-stent restenosis (A, black arrow), which is clearly depicted by longitudinal OCT assessment (B). The dashed white arrows correspond to the cross-sectional images in C, which reveal covered (white arrow) and uncovered (red arrow) stent struts. D, Heterogeneity of the pixel intensity in an area of neointimal hyperplasia (homogeneous high-intensity signal-intensity tissue, red arrows; and heterogeneous predominantly low-intensity signal-intensity tissue, white arrows). Furthermore, intraluminal thrombus is revealed (D, blue arrow). After stent placement, angiography shows a good result (E). OCT cross-sectional image (F) depicts 2 layers of stent struts: the previously deployed (blue dashed arrow) and the recently implanted stent (blue solid arrow).
Patient D
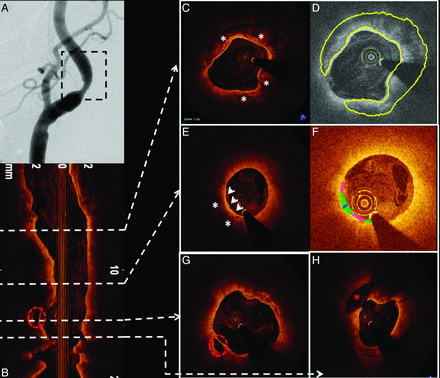
Patient D was a 79-year-old woman with asymptomatic right ICA stenosis of moderate severity by duplex examination (ICA peak systolic velocity = 301 cm/s; diastolic velocity = 55 cm/s; ICA/CCA ratio = 3.8). Diagnostic carotid angiography revealed an ulcerated 58% (NASCET) stenosis at the origin of the right ICA (Fig 5 A). OCT was performed to assess the lesion, which had only marginal conventional criteria for revascularization. A large complex plaque with extensive calcification (Fig 5C, -D) and OCT-derived NASCET measurements of only 40% diameter stenosis was noted. Most important, images also demonstrated large regions of lipid with an extensive area of TCFA (Fig 5E, -F) and 2 sites of significant plaque ulceration (Fig 5G, -H). On the basis of these pathologic findings and the patient's age, she was referred for carotid endarterectomy.
OCT demonstration of ICA calcified plaque, TCFA, and plaque ulceration. A, Carotid angiogram shows a mild-to-moderate narrowing of the right ICA (region of interest, black dashed square). OCT longitudinal view of the region of interest is revealed in B. The white dashed arrows point to their respective cross-sectional images. C, Highly calcified plaque is shown (white asterisks). The same cross-section is represented in D, using semiautomated software for calcium detection and quantification (highlighted in yellow). E, TCFA. A signal-intensity-poor region (white asterisks) corresponding to lipid presence is identified below the fibrous cap (white arrowheads). Automated fibrous cap thickness detection F, Fibrous cap heterogeneity in a color-coded display (pink, <65 μm; green, 65–149 μm; blue, 150–220 μm). G, Red dashed circle represents cavity formation due to plaque ulceration. This area is also easily identified in the longitudinal view (B). Another cavity (H, red asterisk) located more proximally is also noted.
Discussion
Ischemic stroke is largely an embolic disease. Nonetheless, current standard-of-practice guidelines suggesting that carotid revascularization with endarterectomy or a stent procedure19 is beneficial in asymptomatic patients are largely based on North American20 and European21 trials. Both trials, limiting enrollment to patients with at least 60% angiographic stenosis, reported only modest benefit of an operation over medical management, with a 3-year absolute risk reduction of approximately 3% and no definite correlation between the degree of stenosis and outcomes.
Indeed, several investigators have reported data suggesting that risk of stroke is poorly defined by the degree of stenosis but strongly related to histopathologic findings in carotid plaques. Spangoli et al22 examined surgically removed plaques from patients without symptoms versus those with transient ischemic attack or cerebrovascular accident. They stressed that the presence and severity of clinical events was significantly correlated with thrombus and cap inflammation in ruptured plaques, after assessing endarterectomy specimens in a cohort with similar degrees of angiographic stenosis. Similarly, in another histopathologic evaluation, a higher percentage of macrophage-rich areas and number of T-cells per square millimeter in symptomatic-versus-asymptomatic patients with similar degrees of carotid narrowing was described.23
Moreover, using multidetector CT angiography to examine symptomatic atherosclerotic plaques in patients with anterior circulation disease, Homburg et al24 found that culprit plaque ulcerations were equally distributed between patients with more than and less than 50% carotid stenosis. Further evidence that symptoms from carotid artery disease are correlated with plaque histopathology has been provided by a report that established correlation between plaque ulceration, intraluminal thrombus, symptoms, and cerebral microemboli as detected by transcranial Doppler in patients with similar degrees of angiographic stenosis.25 Redgrave et al,26 in the histopathologic study of 526 carotid endarterectomy specimens from recently symptomatic stenosis, found a high prevalence of cap rupture, large lipid core, and attenuated macrophage infiltration, highlighting the fact that these markers for plaque instability were similar to those previously described in unstable coronary artery plaques.
OCT is an emerging technology in the cardiac catheterization laboratory. Its accuracy in measurements of vascular dimensions,27 in the characterization of atherosclerotic plaques,11,28 and in the identification of thrombus16 and plaque inflammation15,29 has been well demonstrated; therefore, it enables imaging of structures that have a potential causative role in cerebrovascular ischemia (eg, thrombus, TCFA with plaque rupture, and inflammation).
In this case series, we report the use of FDOCT to assist in the management of 4 asymptomatic patients in whom a proper course of action was not readily apparent after diagnostic studies that included conventional angiography. Patient A underwent OCT evaluation to clarify abnormal but nonspecific findings on a carotid angiogram following carotid stent placement, with resultant findings that included marked tissue prolapse through the stent struts and the presence of an otherwise undetectable ruptured TCFA. The 3500-patient CAPTURE (Carotid Acculink/Accunet Post-approval Trial to Uncover unanticipated or Rare Events) carotid stent registry, reporting a 30-day stroke rate of 4.8%, found that only 23% of strokes occurred intraprocedurally,30 raising the possibility that at least some of the ipsilateral postprocedural strokes following carotid artery stent placement might be related to issues surrounding tissue prolapse through stent cell struts. In patient A, OCT findings resulted in a decision to place a second (closed cell) stent in the target lesion to treat tissue prolapse. Moreover, another change in procedural strategy, specifically stent length, could have been considered to achieve full coverage of the segment containing the ruptured TCFA had OCT been performed before stent deployment in this case.
Intervention was, however, deferred in patient B, despite inexplicably high ICA velocities that could otherwise justify revascularization, on the basis of OCT documentation of <60% stenosis and the absence of any features thought to suggest plaque instability by pathologic criteria. Revascularization was recommended for patient D, after an OCT evaluation of “borderline” carotid duplex and angiographic data demonstrated findings that have been associated with higher risk in the pathology literature.31 Patient C, despite the presence of “low-risk” clinical (restenosis) and angiographic (60% stenosis) markers, was treated with carotid stent placement when an unanticipated large thrombus burden was detected by OCT. This case as well demonstrates the ability of OCT to differentiate normal- and abnormal-appearing tissue on the basis of signal intensity coupled with homogeneity and smoothness of tissue contour. Although these findings have not been previously described in the carotid arteries or validated by OCT, echolucent tissue, described as a “black hole” in intravascular sonography studies after intracoronary brachytherapy and drug-eluting stent implantation,32 is composed of acellular/hypocellular necrotic tissue, scattered in an extracellular matrix rich in proteoglycans but also containing T lymphocytes and foam cells.33 On the basis of known optical properties of different materials, tissue depicting poor signals on OCT images is likely composed of fibrin or organized thrombus,34 though highly organized proteoglycan could also provide similar images.35
Yoshimura et al36 initially described the use of carotid OCT with balloon occlusion to clarify an ambiguous carotid angiogram in 2010. More recently, the use of OCT to evaluate carotid artery stent placement was reported in 7 patients for whom the authors emphasized the necessity of proximal balloon occlusion (complicated by symptomatic cerebral ischemia in 1 patient) to obtain acceptable images.37 Furthermore, an early experience with OCT in carotid plaque characterization was reported in a brief communication that compared OCT with other imaging modalities.38 Most recently, a 30-patient cohort undergoing scheduled carotid artery stent placement was studied before and following stent placement with TDOCT.39 Again, all patients were imaged after proximal balloon occlusion. Conversely, our group acquired OCT images with hand injections of contrast without proximal balloon occlusion. FDOCT technology, with a much faster pullback speed (up to 25 mm/s) compared with TDOCT, enables adequate blood clearance, without the necessity of proximal balloon occlusion. Furthermore, its wider FOV (∼10 mm) allows imaging of larger arteries.10 In our series, only 13% of all the cross-sections were not suitable for analysis because of blood clearance artifacts or inadequate FOV.
In contrast to these earlier studies, our group also reports the use of data obtained by preintervention FDOCT in clinical decision-making in patients with ambiguous angiographic or noninvasive data. No complications were reported in this case series.
The present cases, although not conclusive, provide important clinical insights. First, the feasibility of interrogating the carotid vasculature by using high-resolution FDOCT imaging without the need for complex technical adaptations for blood displacement or interruption of carotid flow is shown. Second, these cases are, to the best of our knowledge, the first use of FDOCT to assess plaque morphology and guide, albeit empirically, decision-making before planned intervention in human carotid arteries in vivo. Finally, this report also describes the use of “next -generation” analytical imaging software with automated detection and quantification of vascular disease that capitalizes on the 3D nature of FDOCT images.
Several points of caution should be noted in the interpretation and use of data presented in this case series. First, carotid artery instrumentation may carry a risk of embolization. While no signals of safety issues in excess of those associated with diagnostic carotid angiography have been reported to date in small series of carotid intravascular sonography and OCT patients,9,36⇓⇓⇓–40 the risk versus benefit of this technique will remain uncertain until studies that are appropriately powered to assess safety and efficacy end points are available. Second, we assumed that OCT histopathologic correlations that have been extensively validated outside of the carotid circulation11⇓⇓⇓⇓–16,34,35 are also valid in the carotid circulation. Finally, limited tissue penetration, particularly in lipid-rich plaques, precludes the interpretation and quantification of underlying tissue layers.
In this case series, FDOCT revealed unexpected findings in virtually all patients including marked tissue prolapse through an open-cell carotid stent and the demonstration of ruptured TCFAs and thrombus in lesions defined as “low-risk” according to current clinical criteria. These unexpected findings suggest that further studies are needed to evaluate the role of FDOCT in the assessment of asymptomatic carotid artery disease.
Conclusions
The present study demonstrates the technical feasibility of FDOCT without balloon occlusion for the assessment of extracranial carotid artery disease. The unique features of this technology, which allow the rapid acquisition of images with unprecedented resolution, make FDOCT a promising tool to help clinicians understand and manage patients with asymptomatic carotid artery disease.
Footnotes
Disclosures: Michael R. Jones—RELATED: Consulting Fee or Honorarium: St. Jude Medical, UNRELATED: Payment for Lectures (including service on Speakers Bureaus): St. Jude Medical Speakers' Bureau. Curtis A. Given II—UNRELATED: Payment for Lectures (including service on Speakers Bureaus): ev3, Comments: I am a proctor and on the Speakers Bureau for Onyx 18, 34, and HD500. Marco A. Costa—UNRELATED: Consultancy: St. Jude Medical, Grants/Grants Pending: St. Jude Medical,* Patents (planned, pending, or issued): a patent of University Hospitals for OCT Software,* OTHER RELATIONSHIPS: prior consultant/speaker honorarium fees by Boston Scientific, Cordis, Medtronic, Abbott Vascular. Hiram G. Bezerra—UNRELATED: Consultancy: St. Jude Medical. *Money paid to the institution.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received October 5, 2011.
- Accepted after revision December 7, 2011.
- © 2012 by American Journal of Neuroradiology