Abstract
SUMMARY: As stroke is one of the leading causes of death and long-term morbidity worldwide, the research community has studied cardiac embolic sources, as well as vessel wall pathologies. For the latter, attention has been focused on defining morphologic tissue features associated with catastrophic events stemming from the carotid artery. Multiple noninvasive imaging modalities are currently being used to image and classify carotid atherosclerotic plaques, such as MR imaging, CT, and sonography, in an effort to provide clinically relevant predictive metrics for use in patient risk stratification and to define appropriate treatment options. This article compares and contrasts these existing clinical imaging modalities along with discussion of a new endovascular technique originally developed for cardiology, OCT, with which 3D comprehensive high-resolution images of the arterial wall can be acquired.
ABBREVIATIONS:
- AHA
- American Heart Association
- CE
- contrast-enhanced
- CI
- confidence interval
- HR
- hazard ratio
- MDCTA
- multidetector row CT angiography
- MRDTI
- MR direct thrombus imaging
- OCT
- optical coherence tomography
- TOF
- time-of-flight
Stroke is a leading cause of death and the major cause of long-term disability worldwide.1⇓⇓–4 Approximately 80% of strokes are ischemic and are thought to occur through embolic events or stenosis of cerebral arteries. More specifically, 20%–30% of cerebral infarction has been correlated to carotid atherosclerotic plaque and artery stenosis.5,6 Morphologically, features such as carotid artery intima-media thickness,7⇓–9 rupture-prone plaques with thin fibrous caps and lipid cores,10⇓–12 and ulcerated plaques13,14 have all been correlated with an increased risk for stroke. Such high-risk features may be present even in patients with mild or moderate degrees of carotid stenosis. Conversely, plaques with high superficial calcium content have been associated with a lower risk for stroke.15 Noninvasive imaging techniques have been developed to extract these carotid plaque features by using sonography,16⇓–18 MR imaging,19⇓⇓–22 and CT.14,19,23,24 The characterization of carotid artery plaque presents an opportunity to quantify a patient's risk of cerebrovascular events and may be used to improve the therapeutic decision-making process, such as carotid endarterectomy or angioplasty and stent placement. We present the current state-of-the-art, reflect on future high-resolution quantitative vulnerable carotid plaque imaging techniques with discussions of their potential as predictors of the occurrence of cerebrovascular accidents, and refine our understanding of stroke risk stratification.
Atherosclerotic Plaque Identification
Atherosclerosis is characterized by progressive thickening of the arterial wall, with the deposition of cholesterol, inflammatory cell infiltration, extracellular-matrix formation, and thrombosis.25 A large portion of the general population exhibits nonsymptomatic atherosclerosis; in late-stage disease, symptoms become apparent and are usually caused by arterial stenosis and/or embolic events. Progressive atherosclerotic plaque formation can limit blood flow to key organs, such as the heart or brain, impairing function, while acute or sudden plaque rupture and subsequent thrombosis can cause myocardial infarction or stroke.
Traditional diagnosis and classification of atherosclerosis was possible only in late-stage disease formation, where arterial occlusion or stenosis were revealed through angiography or organ perfusion measurements.26,27 Due to recent advancements in medical imaging, new opportunities exist to explore and assess both the arterial morphology of blood vessels and the actual stage of atherosclerosis formation within the lumen wall itself by using higher resolution modalities. Pathology is the current criterion standard of atherosclerosis classification (Table 1). Therefore to critically evaluate the ability of these new medical imaging modalities to quantify and possibly predict ischemic or hemorrhagic stroke, one must determine which characteristics of lesion formation will result in evidence-based improved cerebrovascular care.
Phases of the formation of atherosclerotic plaque according to the American Heart Associationa
From Table 1, several characteristics become apparent as key metrics for the identification and staging of atherosclerotic plaque progression. Specifically, at the cellular level, if the presence and distribution of macrophages and/or foam cells could be detected, early lesion formation could be identified and tracked with time to determine the progression of cardiovascular disease. However, one could argue that because type I/II lesions are present in almost the entire general population, this identification may provide limited insight into its ability to predict the occurrence of a stroke. Additional markers that carry a significant amount of importance include the composition, physical size, and shape of the vessel wall. This presents a challenge for all currently available imaging modalities, in that a potential solution must be capable of detecting submillimeter abnormalities, provide wide-field scanning ability, and also limit the associated cost burden required to image large populations.
CT
Previously, DSA had proved useful for the evaluation of the atherosclerotic disease state because the detection of angiographic ulceration and abnormalities are strong candidates for the prediction of plaque rupture. DSA was originally used for the detection of carotid stenosis and ulceration,29 but recent advances in CTA have supported the opinion that CTA is superior in the detection of carotid dysfunctions, such as plaque irregularities and ulcerations. Saba et al30 recently reported the specificity and sensitivity in the detection of ulcerations via MDCTA to be 99% and 94%, respectively.
de Weert et al23 have taken the detection of plaque ulceration 1 step further in a prospective study that was composed of patients with ischemic cerebrovascular disease in which the plaque surface morphology, severity of stenosis, and cardiovascular risk factors were related to the type of cerebrovascular symptoms. Four hundred six consecutive patients with ischemic cerebrovascular disease (ie, transient ischemic attack, amaurosis fugax, stroke) were first evaluated for the presence of atherosclerotic plaque, which subsequently was classified as smooth, irregular, or ulcerated (Fig 1). It was observed that irregular and ulcerated plaques were significantly different (P < .001) between the lowest degree of stenosis (0%–29%) and the higher degrees of stenosis (30%–99%). Therefore complex plaques were more frequent in a higher degree of carotid artery stenosis. Although promising, their results also demonstrated limitations with this approach because atherosclerotic plaques were found in both symptomatic (55%) and asymptomatic (56%) carotid arteries with a slightly higher presence of complex plaques in symptomatic patients (25% versus 18%, respectively). If MDCTA is to be used as a predictor of acute stroke, additional CTA imaging metrics are required to separate high-risk patients so that they receive appropriate medical attention.
Multiplanar reformat images (1 mm thick). A, Smooth atherosclerotic carotid plaque surface. B and C, Irregular plaque surface. D, Atherosclerotic carotid plaque ulceration. Arrow in D demonstrates extension of contrast material beyond the vascular lumen into the surrounding plaque. Reproduced with permission from de Weert et al. Stroke 2009;40:1334–40.23
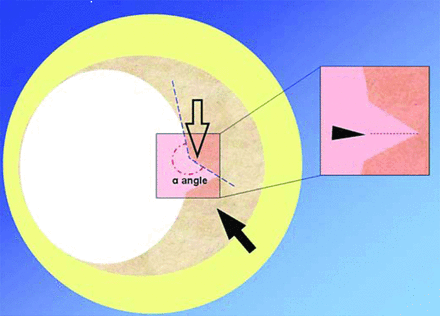
Several authors speculated that atherosclerotic lesions which contain a large necrotic core that is separated from the lumen by a fissured fibrous cap represent an increased stroke risk.31,32 Saba and Mallarini24 presented a retrospective imaging review (n = 147) of an MDCTA technique to evaluate the association between the presence of a fissured fibrous cap and ipsilateral symptoms. They used 4 imaging criteria to define the fissured fibrous cap; all 4 criteria needed to be present to confirm the formation of a fissured fibrous cap (Fig 2). Their results indicated a weak trend in the observation of more frequent fissured fibrous caps when the carotid artery exhibited a higher degree of stenosis:33 11.76% in class IV stenosis, 16.92% in class Va stenosis, and 20% in Class Vb stenosis. Of the 147 patients, 15 were excluded due to poor MDCTA image quality. Of the remaining 132 patients, in 36 symptomatic patients, 12 (33.3%) ipsilateral fissured fibrous caps were detected; and in the 96 asymptomatic patients, 11 (11.5%) fissured fibrous caps were detected. This finding resulted in the presence of a statistically significant (P = .003) association between the detection of a fissured fibrous cap and ipsilateral symptoms. The authors concluded with the observation that fine surface features of atherosclerotic plaques could play an important role in risk stratification and merit additional investigation.
Representation of a carotid artery with a fissured fibrous cap. The 4 imaging criteria are depicted as follows: 1) presence of an “in-plus” image opening into a fibrous cap (open arrow); 2) a depth of ≤1 mm (arrowhead); 3) the angle of ≥230° with respect to the lumen; and 4) the presence of atherosclerotic plaque into which the “in-plus” image projects (arrow). Reproduced with permission from Saba and Mallarini. Cerebrovasc Dis 2009;27:322–2724 and S. Karger, AG, Basel.
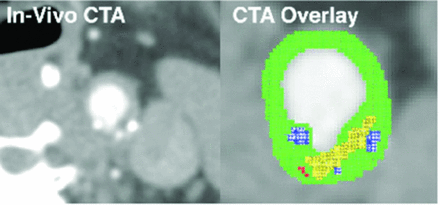
Because the progression of atherosclerosis is a complex biologic event, additional imaging metrics are required to fully identify a patient's risk for stroke, based not only on luminal surface features but also on subsurface tissue morphology. Building on their previous MDCTA studies, which quantitatively identified structures such as carotid plaque calcification, large lipid cores, detection of ulcerations, and fissured fibrous cap thickness measurements, Wintermark et al14 subsequently used these imaging metrics to retrospectively investigate their association with the occurrence of ischemic stroke.34 The study consisted of a consecutive series of 136 patients, of whom 40 patients with carotid stroke and 50 patients without carotid stroke were considered for MDCTA image analysis, after patient review. The image postprocessing consisted of an automated classifier computer algorithm that had been previously validated, which extracted features of the 3 cm on each side of the carotid bifurcation.14 The algorithm segmented the inner and outer contours of the carotid artery wall and distinguished different components, such as lipids and calcium. These are displayed in a color overlay on the CT dataset for ease of visualization (Fig 3). The algorithm then quantifies, in 3D, several features such as lumen area, carotid wall volume, number of calcium clusters, number of lipid clusters, fibrous cap thickness, and so forth. This technique demonstrated that a small number of carotid wall MDCTA features (ie, increased wall volume, greater number of lipid clusters, and lipid clusters that were closer to the lumen) were significantly associated with patients with acute carotid stroke.
In vivo CTA image of the common carotid artery and automated classification computer algorithm-derived overlay highlighting the lipid-rich necrotic core in yellow, calcification in blue, blood products in red, and the remaining connective tissue in green. Reproduced with permission from Wintermark et al. AJNR Am J Neuroradiol 2008;29:875–82.14
CT has demonstrated its clinical utility for accurate characterization of the degree of carotid stenosis but has limited carotid wall characterization outside of calcium content as a predictor of stroke. Advantages of MDCTA are that it is part of the standard of care for patients admitted with cerebrovascular disease, is widely available within hospital infrastructure, and with additional multicenter validation, presents an opportunity to improve stroke risk stratification and provide new perspectives for preventative treatment.
MR Imaging
It is generally accepted that plaque rupture and subsequent luminal thrombosis formation may be the most important mechanisms leading to acute ischemic stroke.31 High-resolution MR imaging has become an integral component for the evaluation of transient ischemic attacks and strokes and seems to be particularly useful for investigating the morphology of the carotid artery. Specific examples include the ability to discriminate large lipid cores with macrophage infiltration, thin and fragile fibrous caps, calcified nodules, characterization of intraplaque hemorrhage, and acute thrombosis.35,36 Serfaty et al37 have proposed and published an adapted AHA classification of atherosclerotic plaque specifically for MR imaging. This classification (Table 2) highlights the ability of MR imaging to organize the plaque on the basis of composition and morphology, specifically the detection of small and large lipid core differences and the presence of calcifications and plaques with hemorrhage or thrombosis.
Conventional AHA classification of atherosclerotic plaques adapted for MR imaging
Various MR imaging signal-intensity acquisition techniques have been investigated to optimize the detection of the lumen wall in carotid arteries in combination with bolus injections for contrast enhancement. 3D TOF MR angiographic acquisition with thin sections, small voxels, and short TEs has been used to achieve higher resolution images. However, this technique may still have signal-intensity loss due to unpredictable transit time, which can be particularly troublesome in stenotic arterial segments, where the residual lumen can be very small; the size makes accurate lumen border detection and composition quite challenging.38 An alternative approach to quantify stenotic regions consists of a time-resolved 3D acquisition technique that repeatedly images a vessel of interest during the passage of a bolus of contrast agent. This technique has been termed time-resolved imaging of contrast kinetics and provides 3D datasets with a temporal resolution of 2–6 seconds, yielding an angiographic series similar to standard angiography.39,40 Therefore, this technique can identify vessels that may opacify late in the imaging sequence, which may prove to be advantageous over non-time-resolved contrast-enhanced techniques because the contrast enhancement usually provides a single snapshot during the peak of contrast-bolus perfusion. However, diseased or stenotic vessels with slow flow rates may opacify late; therefore, these important vessels (or vessel defects) may still not be optimally visualized.
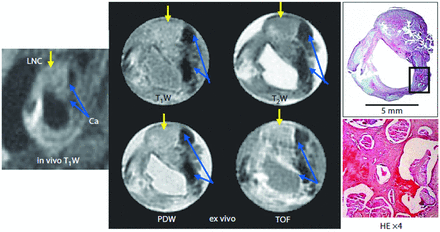
Although MR imaging is generally well-suited for imaging the soft-tissue components of carotid vulnerable plaques, can these measurements be used to predict subsequent stroke? Gao et al22 have recently released a study in which high-resolution CE-MRI was compared with subsequent histologic sections from specimens to assess the accuracy of MR imaging (Fig 4), followed by an evaluation of the relationship between the carotid plaque characteristics and their various types of associated strokes.
Carotid artery bifurcation vulnerable plaque of in vivo and ex vivo samples as seen on MR imaging (T1W, TOF, proton density-weighted [PDW], and T2-W) along with the corresponding matched H&E histologic cross-section. The external carotid artery is seen just above and to the right of the internal carotid artery. The external carotid artery was occluded with a large lipid necrotic core (LNC). Areas of dense calcification (Ca) appear as a dark region from the 1- to 5-o'clock position on MR imaging and the histologic cross-section of the internal carotid artery. The H&E-stained histology section confirms the presence of a superficial calcified nodule. Reproduced with permission from Gao et al. Cerebrovasc Dis 2009;27:345–5222 and S. Karger, AG, Basel.
Their single-center prospective study consisted of 102 consecutive patients, in which the patients were assigned to 4 different categories according to the degree of carotid artery stenosis as measured by sonography: 50%, 51%–69%, 70%–89%, and 90%–100%. The patient population was then further classified into 4 groups on the basis of MR imaging atherosclerotic lesion observations; type III, invulnerable plaques; types IV−V, lipid/necrotic core, fibrous cap, possible calcification; type VI, surface defect and intraplaque hemorrhage; and type VII, calcified plaques. Their findings demonstrated that 45 patients had carotid stroke and 55 patients (2 patients were removed from study due to poor image quality) had lacunar stroke and asymptomatic infarction. They observed a significant correlation (P < .001) between the presence of mild-to-moderate stenosis (≤70%) and types IV−V vulnerable plaque. In patients with carotid stroke (n = 45), 30 had thin or ruptured fibrous caps, twice the number that exhibited thick and intact fibrous caps. These results were consistent with previous literature and indicate that high-resolution CE-MRI may provide useful information for the appropriate selection of candidates for invasive treatment and that the identification of morphologic mechanisms may, in fact, be predictive of stroke.
Takaya et al12 also used MR imaging as a powerful tool to quantify carotid plaque on the basis of the plaque characteristics as a predictive measure of future ipsilateral cerebrovascular events.12 One hundred fifty-four consecutive patients who had asymptomatic 50%–79% carotid stenosis (measured by sonography) were scanned by MR imaging at the beginning and every 3 months to a mean follow-up time of 38.2 months to identify symptoms of cerebrovascular events. Significant associations between the baseline MR imaging and subsequent symptoms included the presence of a thin or ruptured cap resulting in an HR of 17.0 (P ≤ .001), and the occurrence of intraplaque hemorrhage producing an HR of 5.2 (P = .005). These results demonstrated the potential usefulness of MR imaging to provide predictive measurements to assess patient risk and form the bases for large multicenter studies to confirm these results.
Murphy et al41 have shown that MR imaging has the ability to discriminate between different stages of thrombus and hemorrhage formation, via the formation of methemoglobin during the acute/subacute phase, resulting in the signal-intensity increase of the T1WI sequence. This technique was used for in vivo detection of carotid plaque in the ipsilateral arteries of symptomatic patients with suspected stenosis of the carotid artery. These findings were then compared with the authors' own contralateral arteries and with those of healthy sex- and age-matched controls.42 An MRDTI scan with positive findings was diagnosed if there was high-signal-intensity content within the wall or lumen of the carotid artery in the proximity of the lesion and 1 cm on either side of the stenosis. This observation (or lack of) was recorded for both the ipsilateral (symptomatic) and the contralateral (asymptomatic) artery for each patient; a sample MRDTI image can be seen in Fig 5. A total of 134 patients were imaged by using MRDTI, consisting of patients (n = 120) with suspected severe carotid artery stenosis (70%–100%) and previous acute cerebral ischemia; 28 control arteries (n = 14) were also imaged. The control subjects presented with no observable high signal intensity in their arteries, while there was a 60% occurrence of high signal intensity in the patients' ipsilateral arteries, suggesting the presence of complicated plaques. This observation was significantly greater in the patients' ipsilateral carotid artery, compared with the contralateral asymptomatic side at 60% versus 36% (χ2, P < .001), respectively.
A, High-signal-intensity material within the right internal carotid artery (symptomatic side). Note a smaller volume of asymptomatic complicated plaque on the left. B, Unilateral right internal carotid disease, whereas no observable high signal intensity is seen on the left (asymptomatic) side. Reproduced with permission from Murphy et al. Circulation 2003;107:3053–58.41
These MR imaging results suggest that the various methods of obtaining carotid plaque contrast may play a potential role in risk stratification, in which the development of appropriate management strategies, based on the identification of problematic plaques, may lead to an improvement in overall patient mortality and morbidity. Some of the hurdles faced by such a carotid MR imaging technology are the cost, availability, and length of imaging time.
Sonography
Doppler sonography offers an inexpensive, noninvasive, portable, and clinically accepted solution for imaging the carotid artery to assess the severity of stenosis, an established risk factor for stroke,43 by quantifying peak systolic and peak diastolic velocity and/or their ratio.16 B-mode sonography of the carotid intima-media thickness has also recently become a noted characteristic for assessment of cardiovascular risk.44,45 State-of-the-art 3D sonographic systems can now also provide measurement of the total plaque volume and area46⇓–48 and lumen wall volume41; preliminary (ex vivo) research has validated the ability of sonography to identify different carotid plaque components.50
Rundek et al51 have recently reported an association between the maximum carotid plaque thickness and the risk of cerebrovascular events. Carotid plaque imaging, via high-resolution B-mode sonography in 2189 subjects, was analyzed; patients were separated into 3 groups: 1) no plaque, 2) plaque with <1.9 mm thickness (value associated with the 75th percentile of the maximum carotid plaque thickness distribution), and 3) ≥1.9-mm carotid plaque thickness. The primary end point of the study was the occurrence of a vascular event such as ischemic stroke, myocardial infarction, or vascular death. Carotid plaque was present in 1263 (58%) subjects, and after a mean follow-up period of 6.9 years, a vascular event occurred in 319 subjects, of whom 121 experienced a fatal or nonfatal ischemic stroke. The authors concluded that maximum carotid plaque thickness is a simple marker of subclinical atherosclerosis; patients with ≥1.9-mm carotid plaque thickness had a 2.8-fold increase in the risk of ischemic stroke (HR, 2.80; 95% CI, 2.04–3.84).
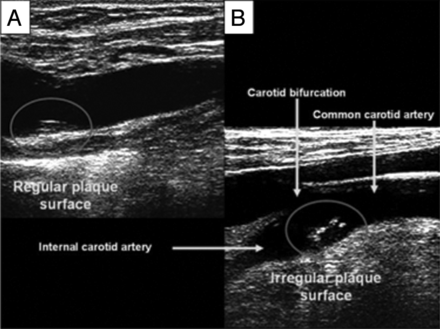
A large prospective study carried out by the Stroke and Critical Care Division of Columbia University recently evaluated the association of carotid plaque surface irregularities and the associated risk of ischemic stroke in patients.44 High-resolution (13 MHz) B-mode sonography was performed in 1939 stroke-free subjects, in whom the presence of carotid plaque was defined as a focal protrusion of >50% of that of the surrounding area and localized to the internal carotid artery bifurcation. The plaque surface was then identified as either regular or irregular (Fig 6), and the subjects were followed for a mean period of 6.2 years, during which time 69 ischemic strokes occurred. It was discovered that there was nearly a 3-fold increase (8.5% versus 3.0%) for ischemic stroke in subjects exhibiting an irregular plaque surface compared with a regular plaque surface. When adjusted for demographics, traditional risk factors, the degree of plaque thickness, and the presence of an irregular plaque were independently associated with ischemic stroke versus the presence of no plaque (HR, 3.1; 95% CI, 1.1–8.5). Therefore, plaque surface irregularities detected by sonography may serve as a useful and easily attainable marker in the high-risk patient. It remains to be seen how sonographic operator dependencies may affect the reproducibility of the plaque irregularity metric.
A, Regular carotid plaque surface. B, Irregular carotid plaque surface. Reproduced with permission from Prabhakaran et al. Stroke 2006;37:2696–701.13
With further clinical investigation, sonography may play an important role as a cost-effective, easy-to operate primary risk-assessment tool, which may appropriately stratify patients for more cost-intensive or invasive procedures, thus improving overall patient care. The development of advanced 3D sonographic techniques may reduce the operator dependency, which has been a traditional drawback of this technique.
Current Imaging Developments: Endovascular OCT
According to the American Heart Association classification of atherosclerotic lesions (Table 1), important markers of vessel wall disease progression include the presence of macrophages, foam cells, and intimal thickening and clear identification of fissures.28 Although the previously mentioned imaging modalities of CT, MR imaging, and sonography can identify key markers of atherosclerosis, these minute cellular markers are below their respective imaging-resolution capabilities. One potential real-time imaging solution to identify these structures is OCT.52 OCT is an imaging technique that can visualize subsurface tissue architecture in highly resolved detail (∼10 μm in tissue). Although, to our knowledge, in vivo OCT carotid artery imaging studies do not currently exist or have not been reported, this technique has had clinical success in the characterization of the coronary artery. To date, OCT characteristics such as lipid content, fibrous cap thickness, and fibrous cap macrophage attenuation have been validated by using histologic controls.53⇓⇓–56
The high spatial resolution of OCT may be an ideal in vivo imaging technology to assess the thickness of the fibrous tissues overlying an arterial plaque. Clinical results have demonstrated that a thin-cap fibroatheroma of <65 μm has a high correlation with the probability of a plaque rupture in the coronary artery.57 Therefore, when designing or implementing a vessel wall imaging standard, the resolution of the imaging system becomes a key component to identify areas of potential plaque rupture. OCT has been shown to clearly identify these key morphologic features, including not only thin-cap fibroatheroma but also the different arterial vessel wall layers, including the intimal layer, the media, and the outer layer. Additionally, the characterization of the different types of plaque elements such as fibrous, calcific, and lipid rich ones and the presence and infiltration of macrophages have been accomplished.53 The macrophage attenuation may be a particularly useful in vivo measurement because it provides a measure of the inflammation within a plaque region.55 Although the process of gaining access to the carotid or coronary arties requires a minimally invasive access point, such as the femoral artery or carotid artery, the fiber-optic delivery of the imaging beam is highly amenable to miniaturization. This allows the use of side-viewing probes (∼300 μm in diameter) to be used in an attempt to reduce the invasiveness and complexity of the imaging procedure.58
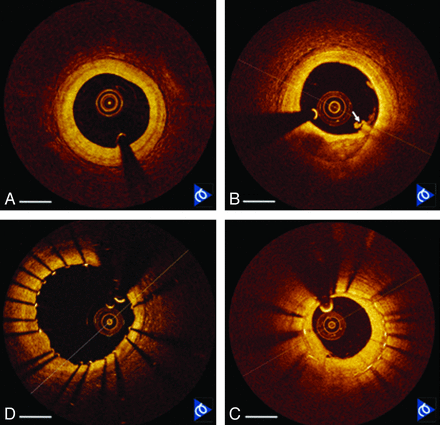
Because OCT is the most recent imaging technique to enter the vascular imaging arena, some may view this technique as too premature for direct comparison with the previously mentioned techniques (ie, CT, MR imaging, and sonography) and its potential use in carotid artery imaging. Evolution to carotid imaging may come from critically analyzing the recent introduction of a US Food and Drug Administration−approved (C7-XR; LightLab, Westford, Massachusetts) intravascular OCT imaging system for cardiology. This system has been used in vivo to image the normal coronary artery (with or without mild intimal thickening), coronary atherosclerotic plaques (with or without calcification), and thrombosis or dissection pathologies. Pre- and poststenting OCT allowed evaluation of stent placement, neointimal hyperplasia, and restenosis, as shown in Fig 7.
Frequency-domain OCT images of in vivo human coronary arteries acquired at 50,000 image lines per second. A, Normal artery with mild intimal hyperplasia. B, Calcium plaque (6 o'clock) with a small thrombus (arrow). C, Freshly implanted stent showing good apposition. D, Previously implanted stent at follow-up showing neointimal thickening and restenosis. Scale bar = 1 mm. Image courtesy of LightLab.
As the OCT technology matures, this technology may become a standard tool to image the coronary artery as a means of detecting and stratifying the progression of atherosclerotic plaque. This has laid the groundwork for a detailed assessment of heart disease and may also be vital in developing an imaging tool to detect and quantify the risk for stroke through investigation of the atherosclerotic plaque in the carotid artery.
Future Speculation and Overall Conclusions
Several noninvasive imaging techniques such as MR imaging, CT, and sonography have shown promise in detecting the fine surface and subsurface morphology of atherosclerotic plaques in the carotid arterial wall. However, these techniques could benefit from higher resolution to image morphologic signatures associated with plaque rupture, including intima-media thickness, macrophage infiltration, and the presence and thickness of a thin-cap fibroatheroma. When these histologically observed markers are present in the carotid artery, they have been correlated with an increased risk for stroke leading to mortality or morbidity. Therefore, should one ask whether the benefits of a high-resolution invasive imaging technique outweigh the potential complications of endovascular carotid imaging?
If we look to previous research concerning the use of a high-resolution system (ie, high-frequency sonography or OCT), specifically in coronary artery imaging, as a preliminary model for success to lay the foundation and extension into carotid artery imaging, one needs to understand and investigate how this imaging scenario could be envisioned. In coronary artery imaging, the proper deployment of a stent is extremely important because the cardiologist uses a balloon-expandable stent in the coronary artery to maintain a minimum luminal area. However, the interventional neuroradiologist uses a self-expanding stent in the carotid artery. Perhaps a more important use of a high-resolution endovascular imaging system may include the characterization of atherosclerotic plaque and how its morphology relates to symptomatic or nonsymptomatic patients with stroke. Cardiologists must also pay attention to the presence and size of any intimal hyperplasia in the coronary artery, whereas due to the much larger diameter of the carotid artery, a similar amount of hyperplasia usually results in limited clinical significance; therefore, we believe this useful coronary imaging metric may be less useful in carotid evaluation. Intracranial stent placement for lesions such as giant aneurysms using the new generation of flow-diversion devices,59 in which the parent vessel diameter is smaller than the proximal internal carotid artery, may benefit from such detailed imaging of neointimal hyperplasia.
At this stage of interventional carotid imaging, we believe the most potentially beneficial implication of an in vivo high-resolution imaging technique would be to characterize carotid plaques before stent placement and to also image poststent placement in an effort to identify any stent-plaque beneficial or detrimental interactions. An initial pre-/post-stent placement prospective study could provide similar quantifiable imaging metrics, such as the thickness of the fibrous cap in the coronary artery, as an assessment of stroke risk.
An additional important point to consider when comparing coronary and carotid artery imaging lies with the practicality of imaging and the intervention in these different vessels. In the past several decades, many new intravascular technologies (eg, intravascular sonography, OCT, and so forth) have been incorporated into the routine care for patients with coronary disease undergoing cardiac catheterization procedures. This is in stark contrast to the carotid artery, in which, for the past several years, there has been an effort to avoid invasive procedures for diagnostic purposes. Therefore, the risk assessment of implementing carotid wall imaging must demonstrate that the benefit of in vivo high-resolution imaging, which currently lies outside the resolution capabilities of existing technologies, including multisection CT, MR imaging, and sonography, must outweigh the risk of invasive diagnostic angiography. Madjid et al60 point out that though the usefulness of locating vulnerable plaque is unproved, prognosis is very valuable to the patient. For example, a patient may postpone travel arrangements or choose elective surgery to address the identified problems. Proper analysis of the risk trade-off from extrapolated data of previous clinical trials and adequate design of new prospective studies remain the key to discussions for evaluating the potential of carotid endovascular imaging.
We believe that a stepwise plan can be designed for high-resolution endovascular imaging, (ie, high-frequency sonography or OCT), during carotid angioplasty and stent placement, in which the primary goal will be immediate assessment of carotid plaque and vessel stenosis pre- and post-balloon angioplasty. A secondary goal would be in vivo characterization of the carotid plaque and comparison with noninvasive imaging. A proper assessment of the risk could then be accomplished as the benefits of this invasive imaging technique are compared with the benefits of endovascular carotid imaging. With this accomplished, one could then envision using these techniques in diagnostic or prognostic applications, where the endovascular imaging method may be integrated into the existing patient management algorithm by using a multimodality approach (ie, sonography, CT, MR, OCT) to identify the vulnerable carotid lesions with high-risk profiles in the low-to-moderate stenosis categories.
Footnotes
-
Disclosures: Thomas R. Marotta—Unrelated: Consultancy: ev3, Comments: proctor for Pipeline device; Employment: private practice. Victor X.D. Yang—Related: Grant: Natural Sciences and Engineering Research Council of Canada,* Comments: Canada research chair; Other: LightLab Imaging,* Comments: in-kind engineering support. *Money paid to the institution.
Indicates open access to non-subscribers at www.ajnr.org
References
- © 2012 by American Journal of Neuroradiology