Abstract
BACKGROUND AND PURPOSE: Cervical steroid injections are a minimally invasive means of providing pain relief to patients with cervical radiculopathy. CT guidance offers many potential advantages. We developed a technique with the patient in the lateral position with a lateral needle trajectory to minimize the required needle depth from skin to target and a near-vertical needle trajectory. The aim of this study was to analyze the cohort for complications, procedural time, and effective radiation dose.
MATERIALS AND METHODS: This was a retrospective evaluation of a single-center patient cohort. PACS images from the procedures were reviewed for needle depth, procedural time, and CTDIvol. An anatomically relevant conversion factor was used to calculate the effective dose.
RESULTS: One hundred sixteen cases from 110 patients were identified. The average patient age was 55 years. There were no complications. In 50% of cases, C5–6 was targeted. The average time was 6 minutes, and the average effective radiation dose, 0.51 mSv (0.21–2.56 mSv). Needle-insertion length from the skin to the target was highly correlated with a need for >3 needle repositioning adjustments and scan series (ρ = 0.52, P < .001) and increased procedural time (ρ = 0.42, P < .001). The angle of needle insertion relative to the floor was significantly correlated with an increased number of needle adjustments for depths >25 mm and a longer procedural time (ρ = 0.29, P = .01) but not for depths <25 mm.
CONCLUSIONS: The lateral patient position with CT guidance is safe and allows use of a short needle in a vertical trajectory. This reduces the number of needle adjustments and imaging series to provide a short procedural time with a low effective radiation dose from the procedure.
ABBREVIATIONS:
- CSI
- cervical epidural steroid injections
- CTDIvol
- CT dose index volume
- DLP
- dose-length product
- ED
- effective dose
Cervical radicular pain affects 0.1% of the population per year.1 Pain relief is obtained with time, physical therapy, and oral medication in most cases. When pain persists despite medical management, cervical steroid injections are a minimally invasive means of providing pain relief to patients with cervical radiculopathy.2,3
Although commonly performed with fluoroscopic guidance, CT guidance offers many potential advantages.4,5 As seen in Fig 1, direct visualization of soft-tissue planes, vital organs and glands, and neural and vascular structures and precise anatomic localization with millimeter accuracy are all features that make CT attractive as a guidance technique. Lack of real-time vascular contrast imaging is a potential limiting feature.6,7
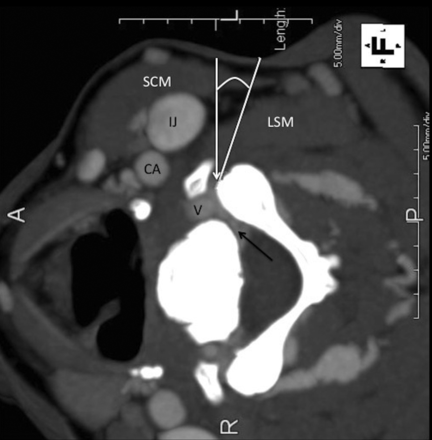
Lateral decubitus position: anatomy and targeting. CT scan with contrast. The white line indicates the deviation from the perpendicular approach; the black arrow, the perineural venous plexus; the white arrow, the target at the anterior margin of the facet. SCM indicates the sternocleidomastoid muscle; IJ, internal jugular vein; CA, carotid artery; V, vertebral artery; LSM, levator scapulae muscle.
The aim of all such procedures, regardless of the technique, is to infiltrate near the exiting nerve root and/or epidural space at the affected level to reduce inflammation believed to cause the patient's discomfort. This is performed while attempting to minimize potential complications, the most feared being inadvertent brain stem or spinal cord embolization.
There are many reports on the safety and efficacy of cervical steroid injections, with various injection techniques. CT-guided injections of the cervical neural foramen are less commonly performed than injections with fluoroscopic guidance. There are clear potential benefits to using the spatial and soft-tissue resolution afforded by CT over fluoroscopic guidance. Several approaches to cervical injections have been described previously.2,3,6⇓⇓–9 A recent report using CT fluoroscopy and a dorsal interlaminar approach for cervical epidural injections demonstrated no complications, 19-minute procedures, and 24 seconds of CT fluoroscopy time.10
In our experience with >10,000 CT-guided spine injections, needle placement is facilitated when the needle depth is minimized and a vertical needle trajectory is used.11 Development of a technique with the patient in the lateral position and a near-perpendicular needle trajectory minimizes needle depth from the skin to the target and creates a near-vertical needle trajectory. In addition, it allows the least amount of muscle tissue to be traversed, the least patient discomfort, and the least potential for bleeding.
We report on results obtained from studying this approach by using lateral decubitus patient positioning, based on a combination of previously reported methods.7,11⇓–13 Although CT guidance, dorsal and laterally directed techniques, and prone and supine patient positioning have been reported, the use of a lateral patient position and procedural time and ED from these methods have not been previously reported in the literature, to our knowledge.
The aim of this study was to describe the procedural time, ED, findings, and complications associated with CT-guided injection of the cervical neural foramen with lateral patient positioning and a lateral skin entry in patients with cervical radiculopathy.
Materials and Methods
Study Design
This institutional review board–approved and Health Insurance Portability and Accountability Act–compliant study is a retrospective case review of a cohort treated with CT-guided cervical steroid injections. The radiology information system was used to identify all CSI from August 2008 to August 2010. Once identified, each patient chart was reviewed, and each procedure was analyzed for positioning, medications, time, CT dose report, and complications. The PACS images from the procedure were analyzed, and the patient chart was reviewed. Board-certified neuroradiologists performed all procedures. Each had a minimum of 5 years' experience performing at least 100 CT-guided interventions annually.
Technique of Cervical Foraminal Injection
All patients provided written informed consent before entering the procedure suite. Risks, including possible cerebral and spinal cord infarct and infection, were discussed. Preprocedural MR imaging was available for all patients and was used to identify target pathology. The patient described his or her radiculopathy, and the combination of clinical symptoms and image abnormality was used to identify the target.
All procedures were performed with intermittent CT guidance by using LightSpeed RT 16/LightSpeed Xtra scanners (GE Healthcare, Milwaukee, Wisconsin) in the helical mode, with 120 kVp, 50 mAs, and 0.8-second rotation time. Operators were in the shielded control room during all image acquisitions. The patient was placed in the lateral decubitus position with the affected side up. The skin was prepped and draped. Scout and reconstructed 5-mm-thick images through the target level were obtained without IV contrast (series 1). Operator preference determined the number of planning images. C2 or the first rib was included to allow accurate counting of interspaces when the scout image was unclear.
A vertical trajectory relative to the target neural foramen was plotted on the CT console. The depth to the foramen was measured. The vertebral artery was identified. The anterior aspect of the ipsilateral articular facet was targeted. Optimal needle position was at the anterior lateral margin of the facet at the affected neural foramen.6 The needle was placed just superficial to the periosteum with its opening directed ventrally. Skin anesthetic was used as necessary.
As shown in Fig 2, a single 2.5-cm, leur lock 25-ga and a 3-mL syringe were used in most cases. The needle was inserted to the predetermined depth. In biopsy mode, the target volume was imaged, and five 2.5-mm images were reconstructed to visualize the needle position relative to target (series 2). The images were reviewed, and deviation from the target was defined. If it was not in a satisfactory position, the needle was adjusted with appropriate trajectory and distance adjustments (series 3). This process was repeated as necessary to ensure precise targeting (series 4 and so forth).
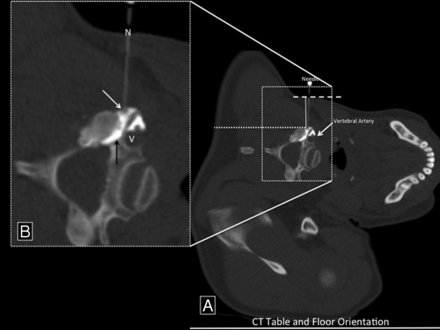
Injection procedure. A, Needle nearly perpendicular to the floor. The dotted line indicates the theoretic dorsal approach to the same target. The dashed line is parallel to the CT table line. The vertical solid line is a plumb line. B, Injection procedure. Enlarged view shows the 3.8-cm, 25-ga needle (N), with the tip at the lateral aspect of the neural foramen (white arrow). Contrast has flowed into the neural foramen (black arrow) and outlined the vertebral artery (V).
Once the needle was in the appropriate position, a slow 1-mL injection of iohexol (Omnipaque; GE Healthcare, Princeton, New Jersey) diluted in 1-mL 1% lidocaine was used in all cases to identify inadvertent direct vessel (arteriole or vein) puncture visually and clinically. After an additional series was obtained and reviewed and after neurologic status was determined to be unchanged, dexamethasone sodium phosphate (Decadron) was slowly injected as a bolus. Patients were monitored by the operator for changes in neurologic status throughout and immediately following the injection procedure. Vital signs were simultaneously monitored by nursing staff as well. All patients returned for 1-month follow-up clinic visits or were contacted by phone.
Data Collection
Each procedure included a planning series of images, followed by series used to visualize needle placement. Subsequent imaging series were obtained following each needle adjustment. Procedural time was measured by using the PACS time stamp on the scout image and the PACS time stamp on the last helical image in the last series of the procedure. Needle distance was measured from the skin surface to the needle tip on the injection image. Estimated distance if using a posterior approach was measured from the dorsal skin surface in line with the target to the needle tip. Needle angle was measured on the PACS workstation from the image in which contrast was injected through the needle. The needle deviation from perpendicular to table surface was recorded.
The percentage of image volume in which the thyroid gland was demonstrated was calculated to determine the ED, which was calculated from the dose report indicated by the CT machine as the DLP. This result was multiplied by a tissue-weighting factor, which was calculated by using the technique described previously and modified in the following way to account for the significant contribution of the thyroid gland to the ED.12
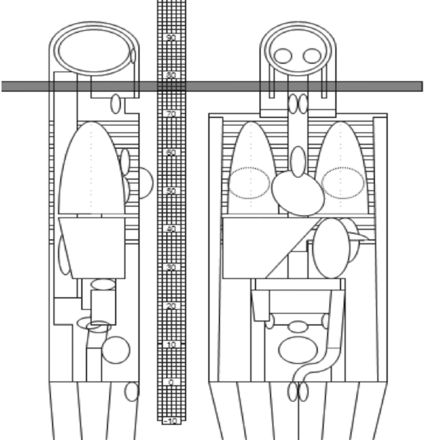
As demonstrated in Fig 3 and the On-line Table, a weighting-factor chart was created by using a Monte Carlo calculation (ImPACT CT Patient Dosimetry Calculator, Version 1.0.2; http://www.impactscan.org) to account for varying amounts of thyroid gland in the field of imaging. The calculations were performed assuming a hermaphrodite phantom and used the International Commission on Radiological Protection 103 weighting factors. The thinnest section thickness allowed in the simulation, 1 cm, was used. ED per DLP (ED/DLP) constants ranged from 1.7 to 12 μSv/mGy-cm over the distance from the skull base to the thoracic inlet. The maximum ED/DLP constant observed when the thyroid was in the scan was 12 μSv/mGy-cm. This was used to calculate the ED for the scan volume including the thyroid gland. In areas where the thyroid was not included, a constant of 2.5 was used for the calculations. Each procedure was analyzed to determine the total number of axial images, total scan length, and total image length with the thyroid gland included. The DLP fraction in which the thyroid was included was multiplied by the thyroid contribution constant,12 and the DLP fraction in which the thyroid was absent was multiplied by 2.5. These numbers were added to obtain the total ED for each series, and then each series was added to obtain the total ED for the procedure.
ImPACT dose software. The shaded area represents a volume of tissue imaged for a scan series. The area is between the skull base and thyroid gland. This was used to calculate constants for the effective dose based on different amounts of thyroid gland in the imaging volume. The grid indicates the section location of the scan and correlates with the On-line Table ImPACT results.
Medical records, including the procedure report, nursing notes, and follow-up office notes, were reviewed for information on complications categorized according to the recommendations of the Society of Interventional Radiology.13
Statistical Methods
Needle-insertion angle and depth were analyzed in an attempt to understand predictors of radiation exposure, which, other than machine settings, were wholly dependent on the number of scan series and imaged volume. The angle of insertion was categorized into 3 groups: <10°, 11°–20°, and >20° deviation from perpendicular. Needle-insertion depth was divided into 2 categories: greater than or less than 25 mm. Attempts at needle repositioningwere divided into 2 groups: <3 attempts and ≥3 attempts. To evaluate correlations with ordinal and dichotomous variables, we used Spearman ρ statistics. Analyses were performed for the group as a whole and for the 2 depth strata. All statistics were performed with the Statistical Package for the Social Sciences for Windows (Version 19; SPSS, Chicago, Illinois), and a 2-tailed α of .05 was used to denote statistical significance.
Results
A total of 116 procedures from 110 patients were identified (6 patients each had 2 procedures during the study period). The average age of the patients was 55 years (range, 30–88 years). There were 41 men and 69 women. There were no immediate or delayed minor or major complications. Fifty-two percent of injections targeted C5–6 (n = 60), and 28% targeted C6–7 (n = 32), with the remaining 20% spread across C2–3 (n = 1), C3–4 (n = 6), C4–5 (n = 15), and C7–T1 (n = 2). A 2.5-cm, 25-ga needle was used in 105 cases. A 25-ga, 7.5-cm spinal needle was needed for 11 patients: 1 injection to C3–4, 4 injections to C5–6, and 6 injections to C6–7. Forty procedures required >3 CT series to place the needle.
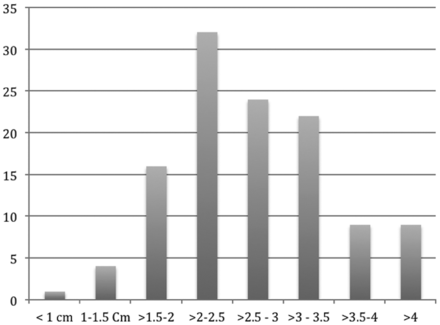
As shown in Fig 4, the average needle depth was 44 mm (9–57 mm) with 50% <2.5 cm in depth and 7.5% >4 cm. The average length of insertion for the 40 procedures requiring >3 CT series was 32.9 mm (Fig 5). The average length of insertion for the procedures requiring ≤3 series was 24.8 mm. The average estimated posterior approach distance was twice the actual lateral approach distance (magnitude range, 1.8–2.3 mm). Procedural time averaged 6 minutes (range, 3–16 minutes). The average time of procedures with ≤3 CT series was 5.1 minutes, while the average time when >3 series were required was 8.75 minutes. Median angle deviation from the perpendicular was 15° on the injection image (range, 0°–48°). For procedures in which ≤3 series were necessary, the average needle angle was 17° from plumb, and when >3 series were required, the average needle angle was 20.4°. The average neck diameter at the needle insertion site was 12.4 cm (median, 11.5 cm; range, 9.5–18 cm).
Histogram demonstrating needle-insertion lengths.
Needle-insertion attempts correlated with target depth and angle deviation from the perpendicular. More insertion attempts increase the procedural time and radiation dose (P < .001).
The average ED was 0.51 mSv (median, 0.38 mSv; range, 0.21–.56 mSv). The thyroid gland was located below the superior endplate of C6 in all cases and below the superior endplate of C7 in 50% of cases. Thyroid content on the images was <10% of the total section volume for procedures targeting C5–6 and above. Only the planning series and not the needle placement series included the thyroid when C5–6 and above were targeted. Because only one-third of the cases were targeted at or below C6–7, the gland was not included in most of the needle-placement images.
Increasing the number of scan series increased the ED from the procedure. Each additional scan series was required after the needle position was incrementally adjusted during targeting. Needle-insertion length from skin to target was highly correlated with a need for >3 needle repositioning adjustments (ρ = 0.52, P < .001) and increased procedural time (ρ = 0.42, P < .001). The angle of needle insertion relative to the floor was significantly correlated with an increased number of needle adjustments for depths of >25 mm and a longer procedural time (ρ = 0.29, P = .01) but not for depths <25 mm.
Discussion
Safely targeting the neural foramen or epidural space is the goal of image-guided transforaminal CSI. Injection procedures are used to deposit steroid solution in the perineural or epidural space, while avoiding intravascular injection. Although it is a generally safe procedure, rare severe complications have been reported with CT and fluoroscopic guidance.8,14
Recently reported techniques focus on CT-guided access to the neuroaxis while minimizing the chance for embolic complications.6⇓–8 We present a cohort treated with a variation on these techniques. A lateral patient position and a near-vertical trajectory with a short 25-ga needle were combined to minimize procedural time and radiation dose. Because proportionally less soft-tissue is crossed in the lateral position, this theoretically decreases the chance that a small muscular artery will be inadvertently pierced. In addition, when comparing the needle-insertion depth by using a lateral trajectory versus a dorsal trajectory within each patient, a 50% reduction in needle depth should also lead to a decreased number of needle adjustments and a decreased radiation dose from the procedure. Procedural time with fluoroscopy has been reported as lasting approximately 15 minutes from initial patient contact to completion.4 We were able to replicate these findings.
Increasing needle-insertion depth and deviation of the angle of approach from the perpendicular were both correlated with more needle adjustments and confirmatory scan series; therefore, both are similarly correlated with an increased ED from the procedure. With intermittent CT guidance, the ED was lower than that reported previously in diagnostic neck CT.15 Adding previously reported dose-reduction techniques might reduce the expected patient dose further.16 Several authors have previously discussed radiation exposures to the operator and patients during image-guided procedures by using fluoroscopy, CT fluoroscopy, and intermittent CT without specifically addressing the ED.9,16 Because all images are acquired with the operator in the CT control room, dose to the operators is zero with this technique.
An anatomically relevant constant was calculated to more accurately define an ED for the procedure. The ED is highly dependent on the presence of the thyroid gland in the imaging volume. A typical ED for a neck CT is 3 mSv, which is 6 times the average in this study. Most of that difference is accounted for by the thyroid gland contribution to the effective dose.15 It is unusual for the thyroid gland to be present above the superior endplate of C6. For those patients in whom a C5–6 or higher injection is contemplated, care taken to exclude tissue caudal to C6 when planning the first cross-sectional dataset can significantly reduce the ED for the procedure. When MR imaging or other imaging is available before the procedure, this may be used to plan a procedure that avoids irradiating the thyroid gland. The average age of the patients in this study was 55 years, which is older than the age at which radiation exposure is most detrimental.17
Limitations of the present study include inaccuracies of the dose-reporting system from the CT console. The DLP measurements are based on the standard adult male 16-cm head phantom. The average neck dimensions in our study more closely resembled a 12-cm-diameter circle, which would result in a higher average dose in the volume of tissue than the CT console value of CTDIvol, which would also result in a higher DLP. We estimate that the CTDIvol would increase by 19% for a 12-cm-diameter phantom by using 2 different methods.18,19
Another limitation in the use of the CTDIvol is that it overestimates the average dose during procedures in which the CT table does not move, such as brain perfusion and certain interventional procedures.20 It has been demonstrated that during simulated brain perfusion procedures, the dose to the skin may vary from 53% to 89% of the CTDIvol, while the dose to the eye lens is approximately 62% of the CTDIvol.21,22 If one assumes an average dose reduction by 75%, the product of this factor by the increase due to the 12-cm neck size results in a net overestimate of the true CTDIvol and DLP of approximately 10%–12%.
The scans in this study used 50 mAs for the planning and subsequent series. This was adequate for visualization of relevant anatomy. Perhaps adopting additional dose-reduction techniques with lower milliampere-second and axial images would further lower the ED of the procedure.16 If a scanner were available that could produce images with <360° scanning range, avoiding the thyroid tissue may be possible in the future.
Conclusions
This series demonstrates the advantages of a near-vertical approach with the patient in the lateral decubitus position with intermittent CT guidance for CSI. Procedures were completed in a short time, with a low radiation dose and without complications.
Footnotes
Paper previously presented at: Annual Meeting of the Radiological Society of North America, November 22–December 2, 2011; Chicago, Illinois.
References
- Received January 22, 2012.
- Accepted after revision March 18, 2012.
- © 2013 by American Journal of Neuroradiology