Abstract
BACKGROUND AND PURPOSE: In our clinical practice, we increasingly use intrathecal contrast-enhanced glymphatic MR imaging to assess CSF disturbances. However, because intrathecal MR imaging contrast agents such as gadobutrol (Gadovist; 1.0 mmol/mL) are used off-label, a thorough understanding of the safety profile is required.
MATERIALS AND METHODS: We performed a prospective safety study from August 2020 to June 2022 of intrathecal gadobutrol, including consecutive patients who received either 0.50, 0.25, or 0.10 mmol. Serious and nonserious adverse events were recorded systematically at 1–3 days, 4 weeks, and >6 months after the intrathecal administration.
RESULTS: The study included 196 patients who received intrathecal gadobutrol, including patients assessed for idiopathic normal pressure hydrocephalus (iNPH, n = 144) or patients examined for other CSF disorders (non-iNPH cohort; n = 52). The intrathecal gadobutrol doses were either 0.50 mmol (n = 56), 0.25 mmol (n = 111), or 0.10 mmol (n = 29). No serious adverse events were observed. Nonserious adverse events on days 1–3 after intrathecal gadobutrol were, to some degree, dose-dependent but mild-to-moderate, including severe headache, nausea, and/or dizziness in 6/196 (6.3%) patients, and they were more common in the non-iNPH than in the iNPH cohort. At 4 weeks, none reported severe nonserious adverse events, and 9/179 (5.0%) patients had mild-to-moderate symptoms. After >6 months, 2 patients reported mild headache.
CONCLUSIONS: The present study adds to the accumulating evidence that intrathecal gadobutrol in doses up to 0.50 is safe.
ABBREVIATIONS:
- GBCA
- gadolinium-based contrast agents
- gMRI
- glymphatic MR imaging
- iNPH
- idiopathic normal pressure hydrocephalus
Since the description of the glymphatic system in rodents in 2012,1 intrathecal contrast-enhanced MR imaging has become of interest for in vivo glymphatic imaging in humans.2⇓⇓-5 A limitation with this imaging technique is that intrathecal gadolinium-based contrast agents (GBCA) are administered off-label. There are concerns regarding the risk of serious adverse events due to acute neurotoxic effects and the risk of deposition of gadolinium in the brain.6 A recent systematic review and meta-analysis7 reported that serious adverse events due to acute neurotoxicity have been observed when MR imaging contrast agents (linear or macrocyclic) are given intrathecally at doses of >1.0 mmol. On the other hand, there are no reports in the literature of serious adverse effects when GBCA are given intrathecally at doses of <1.0 mmol.7
We have previously reported that intrathecal gadobutrol (Gadovist; Bayer Schering Pharma) in doses of ≤0.50 mmol is safe,8,9 though it was difficult to determine the profile of nonserious adverse events because gadobutrol was co-administered with iodixanol (Visipaque; GE Healthcare), a CT contrast agent that may also cause adverse events. In addition, the MR imaging protocol itself with multiple MR imaging acquisitions might add to the patients’ symptoms, and there may be further adverse effects from the spinal puncture. In our previous studies, intrathecal gadobutrol has mostly been given at a dose of 0.50 mmol; only 5 patients received an intrathecal dose of gadobutrol of 0.25 mmol.8 In our clinical practice, we increasingly use intrathecal contrast-enhanced MR imaging (glymphatic MR imaging [gMRI]) in the assessment of CSF disturbances, particularly for assessment of idiopathic normal pressure hydrocephalus (iNPH).10,11 More recently, we have administered intrathecal gadobutrol at lower doses of 0.25 and 0.1 mmol.11
When new indications for drugs are introduced in medicine, there must be an acceptable balance between usefulness and risk. As recently stated by Kanal,12 controlled studies of off-label applications of drugs and devices have always been the mainstay of clinical advances in the medical sciences. Controlled studies of intrathecal GBCA in neuroimaging are, therefore, warranted.
With this background, we performed a prospective study to systematically determine the safety profile of various doses of intrathecal gadobutrol as a follow-up on our previous safety studies.8,9
MATERIALS AND METHODS
Approvals
The study was approved by the institutional review board (2015/1868), the Regional Ethics Committee (2015/96), and the National Medicines Agency (15/04932–7). Patients were included after written and oral informed consent was obtained.
Patients
In this prospective, observational study, consecutive patients undergoing gMRI were included. Imaging was performed on clinical indication in patients admitted to the Department of Neurosurgery, Oslo University Hospital-Rikshospitalet, for the diagnosis of CSF circulation disorders. Patients excluded were those with a history of hypersensitivity reactions to contrast agents, severe allergic reactions in general, evidence of renal dysfunction, pregnant or breastfeeding women, and those younger than 18 years of age or older than 80 years of age. These exclusion criteria have been used since the ethics committee first approved the study in 2015.
Intrathecal Gadobutrol
Under sterile conditions, a spinal puncture was performed at levels L2/L3, L3/L4, or L4/5. Verification of correct needle (22 ga × 3.5 inches) placement in the subarachnoid space was CSF backflow from the puncture needle. Following needle removal, the patients were in the supine position for at least 3–4 hours.
MR Imaging Acquisitions
In this safety study, MR imaging was used to confirm the arrival of intrathecally injected gadobutrol within the intracranial compartment. The MR imaging acquisitions were primarily performed on a 1.5T Aera scanner (Siemens) or a 3T Ingenia MR imaging scanner (Philips Healthcare), and standardized sagittal 3D T1-weighted gradient-echo volume scans were obtained. The MR imaging acquisition parameters and image postprocessing routine have been previously described.3,11
Serious and Nonserious Adverse Events
Study nurses (A.S., I.K., A.H.S.R), not otherwise involved in management of the patients, recorded prospectively and systematically the occurrence of serious and nonserious adverse events during days 1–3 after intrathecal gadobutrol, after 4 weeks, and finally after >6 months.
Serious adverse events refer to the following: any untoward medical occurrence that, at any dose, results in either death, immediate life-threatening situations, requirement of hospitalization or prolongation of existing hospitalization, persistent or evident disability or incapacity, or an important medical event that may jeopardize the subject or may require medical intervention to prevent said outcomes.
Nonserious adverse events did not have the consequences characterizing serious adverse events. The patients were specifically asked for the presence of a defined set of symptoms presenting or being aggravated after intrathecal injection of gadobutrol: headache (mild/moderate/severe), nausea (mild/moderate/severe), dizziness (mild/moderate/severe), itch, warm feeling, paresthesia, visual problems, cognitive difficulties, muscle spasms, discomfort at the injection site, tremor, or other symptoms not specifically requested, independent of the possible cause.
Statistics
Continuous data were assessed by an independent samples t test, and categoric data, using the Pearson χ2 test. The statistical analysis was performed using SPSS, Version 29 (IBM). Statistical significance was .05 (2-tailed).
RESULTS
Patients
From August 2020 to June 2022, one hundred ninety-six patients underwent intrathecal contrast-enhanced MR imaging (gMRI), using gadobutrol in different doses (Table 1). Patients underwent a diagnostic work-up for various CSF diseases: One hundred forty-four patients were examined for iNPH, and 52, for possible CSF diseases other than iNPH: follow-up after subarachnoid hemorrhage (n = 36), follow-up after intracerebral hemorrhage (n = 2), brain tumor (n = 1), noncommunicating or communicating hydrocephalus (n = 3), arachnoid cysts (n = 3), and spontaneous intracranial hypotension leakage (n = 7).
The 2 patient cohorts included (n = 196)
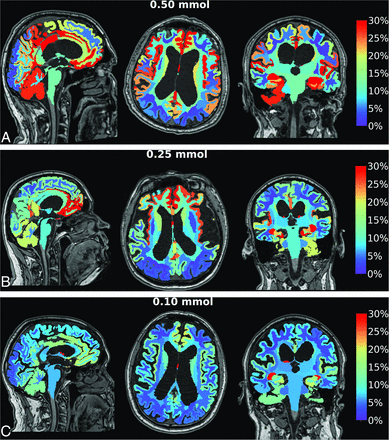
Contrast enrichment in the cranial CSF spaces was verified in all patients by observation of any T1 signal enhancement in the cranial CSF spaces on MR imaging. Figure 1 shows contrast enrichment in the brain after 24 hours in patients with iNPH, depending on the dose of intrathecal gadobutrol, visualized by 1.5T MR imaging. The contrast enhancement in the ventricles is shown in Fig 2.
Enrichment in the brain by gadobutrol, used as a CSF tracer, in patients with iNPH. Axial, sagittal, and coronal MR imaging visualizes dose-dependent brain-wide tracer enrichment 24 hours after intrathecal gadobutrol in the iNPH cohort examined with 1.5T MR imaging at a group level in which intrathecal gadobutrol was given in the doses of 0.50 mmol (n = 19) (A), 0.25 mmol (n = 68) (B), and (0.10 mmol (n = 26) (C). The percentage change in normalized T1 signal at 24 hours is shown on the color bar. In these images, tracer enrichment in the CSF is removed, demonstrating dose-dependent brain-wide tracer enrichment. The tracer enriches the brain centripetally from outside and inward. With 1.5T MR imaging, intrathecal gadobutrol in a dose of 0.10 mmol is not useful for diagnostic imaging, while the doses 0.25 and 0.50 mmol are adequate.
Enrichment in the ventricles by gadobutrol, used as a CSF tracer, in patients with iNPH. Axial, sagittal, and coronal MR imaging visualizes dose-dependent ventricular tracer enrichment 24 hours after intrathecal gadobutrol in the iNPH cohort examined with 1.5T MR imaging at the group level in which intrathecal gadobutrol was given in the doses of 0.50 mmol (n = 19) (A), 0.25 mmol (n = 68) (B), and 0.10 mmol (n = 26) (C) . The percentage change in normalized T1 signal at 24 hours is shown on the color bar. Here, CSF tracer in the brain is removed, showing CSF tracer enrichment of the cerebral ventricles. The high degree of ventricular CSF tracer enrichment is due to the high proportion of patients with iNPH in the study, in whom ventricular reflux of CSF tracer is typical.10
Serious and Nonserious Adverse Events
None of the patients experienced serious adverse events (Table 2).
Distribution of serious and nonserious adverse events days 1–3 or 4 weeks after intrathecal gadobutrol, independent of dose (n = 196)a
The occurrence of nonserious adverse events was higher in the non-iNPH cohort than in the iNPH cohort at days 1–3 (57.7% versus 32.6%, Pearson χ2; P = .002), but not at 4 weeks (Pearson χ2, P = .649) (Table 2). If one specifically addressed the dose of intrathecal gadobutrol, there was no significant difference in the occurrence of nonserious adverse events on days 1–3 between patients with and without iNPH for the dose of 0.50 mmol (P = .678), while for the dose of 0.25 mmol, adverse events were more common in patients without iNPH (P = .005, Pearson χ2 test; Table 3).
Specifications of predominant nonserious adverse events days 1–3 after intrathecal gadobutrol in various dosesa
In the iNPH cohort, the occurrence of nonserious adverse events at days 1–3 was dose-dependent (0.50 versus 0.25 mmol, P = .049; 0.25 versus 0.10 mmol, P = .013), though the distribution of predominant symptoms was not dose-dependent (Table 3). The adverse events were, however, minor. In the iNPH cohort, severe headache, nausea, and/or dizziness occurred in 1/21 (4.8%) patients receiving gadobutrol at a dose of 0.50 mmol, and in 1/94 (1.1%) patients after a dose of 0.25 mmol. The occurrence of predominant adverse events was not dose-dependent (Table 3).
In comparison, in the non-iNPH cohort, nausea and/or dizziness and headache were observed in 4/35 (11.1%) patients after a dose of 0.50 mmol, but in 0/17 patients after 0.25 mmol, with no differences between the doses of 0.50 versus 0.25 mmol (P = .190, Table 3). The occurrence of predominant adverse events was not different for the doses of 0.50 and 0.25 mmol (Table 3).
The difference in the occurrence of nonserious adverse events between patients with and without iNPH at days 1–3 was not statistically different for the doses of 0.50 (P = .427) or 0.25 mmol (P = .056, Table 3).
After 4 weeks, only minor-to-moderate nonserious adverse events were observed, and with no significant dose-dependency (Table 4). In the iNPH cohort, 6/134 (4.5%) patients reported mild-to-moderate headache, nausea, and/or dizziness, while in the non-iNPH cohort, 1/49 (2%) reported mild headache, nausea, and/or dizziness, 1/49 (2%) reported back pain from the spinal puncture, and 1/49 (2%) reported altered taste (Table 4).
Specifications of predominant nonserious adverse effects 4 weeks after intrathecal gadobutrol in various dosesa
At long-term follow-up after >6 months, 2/188 (1.1%) patients reported mild headache. No other symptoms were recorded.
Some missing data in Table 2 were due to not being able to contact patients at these particular follow-up time points.
DISCUSSION
This study adds to previous results that intrathecal gadobutrol in a dose of ≤0.50 mmol is safe. Nonserious adverse events from intrathecal gadobutrol at 0.50, 0.25, or 0.10 mmol were observed, but the profile is favorable.
We have previously reported safety data from 149 patients who received intrathecal gadobutrol from October 2015 to December 2019, and we have concluded that intrathecal gadobutrol at a dose of ≤0.5 mmol is safe.8,9 However, with regard to the occurrence of nonserious adverse events, our previous reports were limited because gadobutrol was commonly co-administered with iodixanol and multiple MR imaging acquisitions, making it difficult to specifically identify which adverse events were caused by gadobutrol alone. In addition, most patients had received gadobutrol at a dose of 0.50 mmol;8,9 the safety profile of intrathecal gadobutrol at a dose of 0.25 mmol was examined in only 5 patients.8 In the present study, we included new patients who had received intrathecal gadobutrol alone, not co-administered with other drugs, from August 2020 to June 2022, demonstrating a more favorable profile of nonserious adverse events than we have previously reported. The present results may, therefore, more correctly describe the safety of intrathecal gadobutrol, while some of the symptoms reported by patients may be related to their underlying disease as well.
The present results add evidence to the growing body of data that intrathecal GBCA in a low dose are safe. A recent systematic review and meta-analysis including 1036 patients from 53 studies concluded that serious adverse events have not been reported after intrathecal GBCA at doses of <1.0 mmol.7 Our previous studies including a total of 149 patients8,9 that supported this conclusion were not included in the above-mentioned meta-analysis.13 The present study of an additional 196 patients further adds support to the previous conclusions. However, GBCA in doses of >1.0 mmol have been reported to cause serious adverse events; even a fatal outcome was reported in a 67-year-old man who accidentally received ProHance (Bracco Diagnostics) in a dose of 2.5 mmol.7 Accordingly, the therapeutic window for intrathecal gadobutrol is narrow. Thus, to prevent accidental overdosage, we always use 1.0-mL syringes to assure that even an accidental overdose cannot exceed 1.0 mmol.
Furthermore, intrathecal GBCA are accompanied by nonserious adverse events, in particular headache. The systematic review and meta-analysis identifying 19 studies including 806 participants reported headache in 120/806 (14.9%) patients after intrathecal GBCA in doses of <1 mmol.7 In comparison, among our 144 patients with iNPH, headache on days 1–3 after intrathecal gadobutrol (0.50 to 0.10 mmol) was observed in 22/144 (15.3%) patients, though it was severe in merely 1/144 (0.7%). Furthermore, among the non-iNPH cohort, 3/52 (5.8%) patients experienced severe headache while 10/52 (19.2%) patients reported mild-to-moderate headache. Thus, the spinal puncture itself may be an important contributor to the headache. Hence, a systematic review and meta-analysis including 31,412 patients showed that the incidence of post-dural puncture headache was 11.0% (95% CI, 9.1%–13.3%), though it was reduced to 4.2% (95% CI, 3.3%–5.2%) when using an atraumatic needle.14 For this reason, we now exclusively use an atraumatic 22-ga syringe for spinal puncture, which expectedly will further reduce the occurrence of headache.
The present data support our previous findings8,9 that the occurrence of nonserious adverse events depends on the underlying disease. For the dose of 0.25 mmol, non-adverse events were more common at days 1–3 in patients without than in those with iNPH. The reason may be that symptoms like headache and dizziness were part of the symptom distribution in patients without iNPH, which included patients with idiopathic intracranial hypertension and intracranial cysts. A similar trend was seen in a previous study in which patients received intrathecal gadobutrol and iodixanol in combination.9
Four weeks after intrathecal gadobutrol, no patients reported severe headache, nausea, and/or dizziness. In both the iNPH and non-iNPH cohorts, symptoms were mild-to-moderate.
Accumulating evidence indicates the benefits of administering GBCA intrathecally. For years, clinicians have used intrathecal GBCA for visualization of CSF leakage in individuals with spontaneous intracranial hypotension.15,16 In our clinical practice, we have also used gMRI for the diagnostic assessment of ventricular reflux (Fig 2) and for estimation of impaired molecular clearance from intracranial CSF spaces and the brain (Fig 1). Hence, we have reported its utility in iNPH,10 idiopathic intracranial hypertension,17 and chronic sleep disturbance.18 Currently, we consider intrathecal contrast-enhanced MR imaging the criterion standard for imaging of glymphatic function, providing information not obtained by other imaging modalities. In a previous study, we showed that intrathecal gadobutrol in a dose of 0.25 mmol (but not 0.10 mmol) is sufficient for gMRI in iNPH using a 1.5T MR imaging scanner.11 When intrathecal gadobutrol is given for the assessment of CSF-to-blood clearance capacity, a dose of 0.10 mmol is sufficient.19
Taken together, the diagnostic benefits of gMRI using gadobutrol in doses 0.5, 0.25, or 0.10 mmol have been demonstrated in several studies. The present data further show a favorable profile concerning the risk of nonserious adverse events after intrathecal gadobutrol at doses of ≤0.50 mmol. In general, the therapeutic index of a drug refers to the balance between risk and benefit or, more specifically, the ratio of the dose in which 50% of subjects experience toxic effects and 50% report effective therapeutic effects. A good safety profile would generally be in the range of a therapeutic index of >10.20 Intrathecal gadobutrol is in this range, given that a potential lethal dose is >2.0 mmol and intrathecal gadobutrol at a dose of 0.25 mmol provides diagnostic information using 1.5T MR imaging.11 Furthermore, 0.10 mmol is enough for estimation of CSF-to-blood clearance19 and possibly for 3T MR imaging.
In addition to the safety profile of intrathecal GBCA, there is a concern about the possible deposition of gadolinium in the brain after intrathecal administration. In this context, IV GBCA are used on-label in much larger body doses. The presently used intrathecal doses of gadobutrol of 0.50, 0.25, or 0.10 mmol are 16, 32, or 80 times lower, respectively, than an intravenous dose of 8 mmol (0.1 mmol/kg in an 80-kg subject). For intravenous use, gadobutrol can be given in doses up to a maximum of 0.3 mmol/kg. IV-administered GBCA also represent a dose to the CSF, even in subjects with normal renal function and an intact BBB.21⇓-23 Several possible leakage sites from blood to CSF have been proposed, such as the choroid plexus,24 ciliary body,25 and cortical veins, at least with increasing age.26 Accordingly, even an intravenous dose of gadobutrol of 0.1 mmol/kg to an individual weighing 80 kg (8 mmol) may provide a substantial dose to the CSF, given that the half-life of gadobutrol in blood is about 2 hours. In older individuals with disrupted BBBs, 1 study estimated that an IV administration resulted in the CSF concentration of GBCA being one-fifth of the IV concentration.23 Therefore, accumulating evidence indicates that the risk of macrocyclic GBCA deposition in the brain via CSF is less than previously assumed.27,28
The major limitation in the present study is that many of the patients, particularly in the non-iNPH cohort, reported symptoms similar to those recorded after intrathecal gadobutrol. Therefore, we cannot definitely conclude which of the nonserious adverse events resulted from intrathecal gadobutrol per se. To this end, we have not given intrathecal gadobutrol to healthy individuals.
CONCLUSIONS
Taken together, intrathecal gadobutrol for gMRI was shown to be safe with no serious adverse events and with a favorable profile of nonserious adverse events. The body of evidence supporting the clinical use of intrathecal GBCA in low doses of ≤0.50 mmol is growing, though such use remains off-label. Further studies should address the clinical risk profile of intrathecal-versus-intravenous GBCA, particularly regarding GBCA deposition in the brain.
Footnotes
This work was supported by grants from Health South-East, Norway (grant No. 2020068) and from the Department of Neurosurgery, Oslo University Hospital-Rikshospitalet, Oslo, Norway.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received January 26, 2023.
- Accepted after revision March 7, 2023.
- © 2023 by American Journal of Neuroradiology