Abstract
SUMMARY: Stroke is a leading cause of death and disability worldwide. Imaging plays a critical role in evaluating patients suspected of acute stroke and transient ischemic attack, especially before initiating treatment. Over the past few decades, major advances have occurred in stroke imaging and treatment, including Food and Drug Administration approval of recanalization therapies for the treatment of acute ischemic stroke. A wide variety of imaging techniques has become available to assess vascular lesions and brain tissue status in acute stroke patients. However, the practical challenge for physicians is to understand the multiple facets of these imaging techniques, including which imaging techniques to implement and how to optimally use them, given available resources at their local institution. Important considerations include constraints of time, cost, access to imaging modalities, preferences of treating physicians, availability of expertise, and availability of endovascular therapy. The choice of which imaging techniques to employ is impacted by both the time urgency for evaluation of patients and the complexity of the literature on acute stroke imaging. Ideally, imaging algorithms should incorporate techniques that provide optimal benefit for improved patient outcomes without delaying treatment.
Stroke is a leading cause of death and disability worldwide. Imaging plays a critical role in evaluating patients suspected of acute stroke and transient ischemic attack (TIA), especially before initiating treatment. Over the past few decades, major advances have occurred in stroke imaging and treatment, including Food and Drug Administration approval of recanalization therapies for treatment of acute ischemic stroke. The primary goal of imaging patients with acute stroke symptoms is to distinguish between hemorrhagic and ischemic stroke. In ischemic stroke patients, secondary goals of imaging before initiating revascularization interventions with intravenous thrombolysis or endovascular therapies include identification of the location and extent of intravascular clot as well as the presence and extent of “ischemic core” (irreversibly damaged tissue) and “penumbra” (hypoperfused tissue at risk for infarction).1⇓–3 In addition, early identification of the stroke etiology or mechanism (eg, carotid atherosclerotic disease, vascular dissection, or other treatable structural causes) is critical to treatment decisions and long-term management.
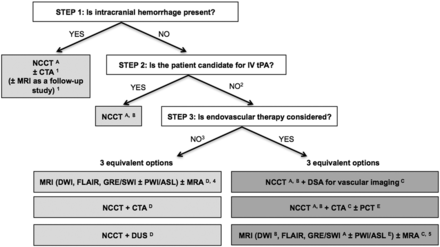
A wide variety of imaging techniques has become available to assess vascular lesions and brain tissue status in acute stroke patients. However, the practical challenge for physicians is to understand the multiple facets of these imaging techniques, including which imaging techniques to implement and how to optimally use them, given available resources at their local institution. Important considerations include constraints of time, cost, access to imaging modalities, preferences of treating physicians, availability of expertise, and availability of endovascular therapy. The choice of which imaging techniques to employ is impacted by both the time urgency for evaluation of patients and the complexity of the literature on acute stroke imaging. Ideally, imaging algorithms should incorporate techniques that provide optimal benefit for improved patient outcomes without delaying treatment. Therefore, it is most practical and efficient to use a standardized imaging approach, with all relevant imaging studies conducted in as few sessions as possible (Fig 1).
Suggested imaging protocols for patients presenting with acute stroke symptoms based on the clinical scenario and the therapeutic options considered and available. Each of the gray boxes represents 1 imaging strategy. In order not to delay treatment, a standardized imaging approach should be used: One imaging strategy (gray box) should be selected, and all imaging studies belonging to this strategy should be performed upfront in as few sessions as possible:
To assess the etiology of the intracranial hemorrhage (CTA for vascular pathologies, such as aneurysms, arteriovenous malformations, vasculopathies; MR imaging for vascular malformations, neoplastic and other pathologies associated with hemorrhage).
Also if the patient is not a candidate for IV tPA (contraindication to tPA, outside the time window for tPA) or if IV tPA failed or it is thought that it may fail.
For patients who are outside the time window for acute reperfusion therapies (>4.5 hours at sites where only IV tPA is being considered; >8 hours at sites where endovascular therapy is considered) and for patients with TIAs, emphasis is on secondary prevention and their imaging work-up should be focused on vascular imaging (CTA, MRA or Doppler-ultrasound [DUS]) to assess carotid arteries as a possible cause of the ischemic stroke, with secondary prevention in mind. If MRA is obtained, it makes sense to concurrently obtain MR imaging with DWI, FLAIR, and GRE/SWI. Echocardiography should also be obtained to assess for cardiac sources.
If available, MR imaging/MRA is the preferred imaging technique for TIA patients.
At institutions where MR imaging is available 24/7 and can be performed within a short time after admission.
To assess for intracranial hemorrhage.
To assess the extent of ischemic core.
To assess the location and extent of the intravascular clot.
To assess carotid atherosclerotic disease.
To assess the extent of viable tissue.
We performed a review of the evidence in the literature on the utility of various imaging techniques in acute stroke and TIA patients to establish best practices with standardization of imaging protocols. We indicated the quality of publications for diagnostic tests and interventions by assigning levels of evidence (Tables 1 and 2). These levels of evidence are based on the Oxford Centre for Evidence-based Medicine−Levels of Evidence.3a The goal of this article is to present practical imaging recommendations for patients presenting with acute stroke and TIA across different practice settings and to provide the rationale and evidence supporting their use. These recommendations are in agreement with the American College of Radiology Appropriateness Criteria.4 We recognize that stroke imaging is a rapidly evolving field and that a number of the recommendations presented are the topic of continued investigation.
Levels of evidence for studies of the accuracy of diagnostic testsa
Levels of evidence for intervention studiesa
Rationale and Imaging Evidence for Patients Presenting with Acute Stroke Symptoms
The initial step in the evaluation of patients with symptoms of acute stroke is to differentiate between hemorrhagic and ischemic stroke (Fig 1). For patients with acute ischemic stroke who are candidates for IV tissue plasminogen activator, a noncontrast CT of the head should be obtained to determine eligibility for treatment. IV tPA can then usually be initiated without waiting for further imaging. In patients under consideration for endovascular therapy, 3 imaging options may be used: 1) NCCT followed immediately by digital subtraction angiography for vascular assessment, 2) NCCT + CT angiography ± perfusion CT (PCT), or 3) MR imaging (±PWI/ASL) + MR angiography at institutions that can offer MR imaging 24/7 without delaying treatment. In patients who are not candidates for IV or endovascular therapy and in patients with TIA, vascular imaging is recommended to guide management for secondary prevention of future stroke.
Imaging Evidence for Assessing Intracranial Hemorrhage
NCCT is the accepted standard-of-care imaging technique for exclusion of intracranial hemorrhage and has been incorporated in the inclusion criteria in randomized clinical trials evaluating the efficacy of IV thrombolysis.4,5 NCCT is often referred to as the “reference standard” for detection of acute intracranial hemorrhage based on reports describing its accuracy with early CT scanners.6,7 However, there are no recent studies that have used a true reference standard, such as surgical or pathologic confirmation, to support level I evidence. Therefore, the sensitivity and specificity of NCCT in detecting intracranial hemorrhage are unknown. The many advantages of NCCT in the emergent setting, as well as the proved benefit of IV thrombolysis in patients selected by NCCT,4,5 have led to its continued widespread use in acute stroke imaging (Table 3).
Advantages and limitations of CT and MR imaging
MR imaging T2*-weighted sequences have been studied for detection of acute and chronic hemorrhage in acute stroke patients. The accuracy of MR imaging techniques for detection of intracranial hemorrhage in the acute stroke setting (within 6 hours) has been reported as likely equivalent to NCCT (level Ib).8,9 Additionally, T2*-weighted sequences (including gradient-recalled echo [GRE] and susceptibility-weighted imaging sequences) have superior accuracy in the detection of chronic microhemorrhages.9⇓–11 In 1 large study as well as a meta-analysis, there was no statistically significant increased risk of symptomatic hemorrhage when patients with a small number of chronic microhemorrhages (<5) were treated with IV thrombolysis (level Ia).12,13 However, the risk of symptomatic hemorrhage in patients with numerous chronic microhemorrhages undergoing treatment with IV thrombolysis is unknown. It is important to recognize that the pivotal CT-based trials proving a benefit for IV tPA likely included patients with multiple microhemorrhages.
If intraparenchymal hemorrhage is present, as in 15% of all strokes, the imaging evaluation in the acute phase may include CTA of the intracranial arteries for evaluation of an underlying vascular malformation.14⇓–16 CTA may demonstrate a “spot sign,” indicative of active bleeding, predictive of hematoma expansion, and strongly associated with poor outcomes.17⇓⇓–20 An MR imaging without and with contrast is sometimes obtained to assess for an underlying neoplastic or vascular mass, or associated microhemorrhages that may suggest amyloid angiopathy, multiple cavernous malformations, or septic emboli among other etiologies. In the acute phase, sensitivity of MR imaging may be limited by mass effect from the hematoma and the complex MR imaging signal of blood products that may obscure subtle enhancing lesions; its sensitivity is improved in the subacute phase once the hematoma has been resorbed.14⇓–16 Please note that the imaging evaluation of patients with aneurysmal subarachnoid hemorrhage is beyond the scope of this article.
Rationale and Imaging Evidence for Acute Ischemic Stroke Patients Who Are Candidates for IV Thrombolysis
Treatment options are considered for patients with acute ischemic stroke without intracranial hemorrhage present on imaging. FDA guidelines for administration of IV thrombolysis include imaging to exclude intracranial hemorrhage and its interpretation by a physician with appropriate expertise, while the completion of this initial imaging within 45 minutes of the patient admission to the emergency department is a Centers for Medicare and Medicaid Services Quality Reporting measure.21⇓–23 There is strong evidence supporting the use of IV tPA as a recanalization therapy to improve clinical outcomes during the 0- to 3-hour time window (level 1a)24⇓–26 and during the 3- to 4.5-hour time window (level 1b).27⇓–29 This benefit is despite an increased risk of symptomatic intracranial hemorrhage after infusion. Overall, there is strong evidence (level Ia) supporting the timely use of imaging of the brain to exclude hemorrhage in patients with the clinical diagnosis of stroke and before initiating IV thrombolytic therapy.4,24 The primary goals of imaging during the 0- to 4.5-hour time window are to exclude the presence of intracranial hemorrhage and assess the presence and extent of ischemic changes. The presence of intracranial hemorrhage (excluding microbleeds) is an absolute contraindication to administering IV thrombolytic therapy. Early signs of ischemia involving more than one-third of the middle cerebral artery territory in the 0- to 6-hour time window have been associated with large infarcted regions, increased risk of hemorrhagic transformation, and poor outcomes and thus constitute a relative contraindication to IV thrombolysis.26,30,31
Imaging in patients who are potential candidates for IV thrombolysis should not delay administration of IV thrombolysis, as “time is brain.”22 Therefore, IV tPA decisions should be made immediately after the NCCT is completed. At institutions that offer endovascular treatment to IV tPA–eligible patients with large-artery occlusion (likely tPA failures), additional imaging can be performed while IV tPA is prepared/administered, to not delay treatment. From a logistics perspective, institutions should develop a standardized imaging algorithm based on their capabilities and interpretation of current evidence. This imaging protocol should be adhered to for all eligible patients to expedite the process and minimize delays in treatment. For instance, if NCCT, CTA, and MR imaging constitute the imaging algorithm selected by an institution to evaluate for potential endovascular candidates, NCCT and CTA should be obtained in 1 imaging session to minimize imaging time. At institutions performing it regularly, the entire multimodal CT evaluation does not delay patient care. Specifically, it does not delay IV thrombolysis, which can be performed directly in the CT scanner once the NCCT is completed and while the CTA and/or PCT are being obtained (level 2b).32⇓⇓–35 Few institutions are able to perform MR imaging studies in the acute setting. Such MR imaging studies are usually performed after the NCCT has been completed (or used as a replacement for it) and are often obtained during or following IV tPA administration.
Imaging Evidence for Detection of Ischemia
NCCT is also used to assess for early signs of infarction, including loss of gray-white differentiation, sulcal effacement, and hyperattenuated clot in the proximal vessels.30,36 NCCT has been reported to have low sensitivity (39%) and high specificity (100%) for detection of ischemic changes (level Ia).30,37 However, the significance of these early signs detected on NCCT has been debated. In the European Cooperative Acute Stroke Studies (ECASS), large infarctions with early swelling had an increased incidence of hemorrhage and poor outcome following thrombolytic therapy.30,37 Conversely, the National Institute of Neurologic Disorders and Stroke rtPA Stroke Trial reported that extensive early signs of infarction on NCCT were associated with stroke severity but not with adverse outcome after thrombolysis.36 However, more recent studies have disagreed and recommended criteria for withholding IV thrombolytic therapy in the 0- to 3-hour time window for definite signs of ischemia involving more than one-third of the MCA territory.38
Detection of early signs of ischemia on NCCT varies among experienced observers,39⇓–41 depending on the size of the infarction, the time between symptom onset and imaging, and the CT window and level settings used. A more objective approach to define the extent of early ischemic changes has been described in the Alberta Stroke Program Early CT Score (ASPECTS), which is a 10-point scoring system of the MCA territory.42⇓–44 Although ASPECTS showed superior interobserver agreement, it only modestly improved accuracy for predicting functional outcome and performed the same as the one-third MCA rule for predicting symptomatic hemorrhage.42 Specifically, an ASPECTS score ≤7 has been shown to predict poor functional outcome with 78% sensitivity and 96% specificity and symptomatic hemorrhage with 90% sensitivity and 62% specificity.23
Of note, the source images from CTA (CTA-SI) have been shown to have increased sensitivity relative to NCCT for detecting ischemic changes, except for infarcts that are small or in the posterior fossa (level II),45,46 though with current technology (rapid CT acquisition), they tend to overestimate the size of the infarct.47 Importantly, CTA-SI maps are strongly dependent on the precise timing of the imaging, which may differ between centers and between individual patients.
MR diffusion-weighted imaging is more sensitive for detecting ischemic changes compared with NCCT (level Ia).48⇓⇓⇓⇓–53 Its sensitivity in detecting ischemia is reported as 99% with a high specificity of 92%.48,50,53⇓⇓⇓⇓⇓–59 In anterior circulation strokes, the DWI lesion volume correlates well with baseline clinical stroke severity, final infarct volume, and clinical outcome (level II).60⇓–62 Although strong evidence suggests that MR imaging is superior to NCCT for confirming stroke within the first 24 hours (level Ia),53 logistical issues related to performing MR imaging in the emergent setting, as well as the proved benefit of CT-based selection in randomized controlled trials, limit the use of MR imaging in the emergent setting (Table 3). Therefore, MR imaging may be reserved for select patients in whom the clinical diagnosis is uncertain or for centers that have MR imaging readily available 24/7 with streamlined protocols to limit imaging time within the standard-of-care guidelines for thrombolytic therapy.
Summary.
In acute stroke patients who are candidates for IV thrombolysis (0- to 4.5-hour time window), NCCT or MR imaging is recommended to exclude intracranial hemorrhage and determine the extent of ischemic changes.4,63,64
The presence of a large acute hypoattenuation on NCCT increases the risk of hemorrhagic transformation after thrombolytic therapy. This is considered a relative, not absolute, contraindication for IV tPA. MR DWI may be obtained for a more definitive estimate of the extent of ischemia, only if this does not delay IV thrombolysis.
The presence of a small number of MR imaging–detected chronic microhemorrhages (<5), in the absence of hemorrhage on NCCT, is not a contraindication to IV thrombolysis. However, the risk of hemorrhage in patients with multiple chronic microhemorrhages (>5) is unknown.
Rationale and Imaging Evidence for Acute Ischemic Stroke Patients Who Are Candidates for Endovascular Revascularization
There is limited evidence supporting the use of intra-arterial thrombolytic agents up to 6 hours. Also, the evidence supporting improved clinical outcomes with first-generation mechanical embolectomy devices up to 8 hours following symptom onset, compared with standard medical care, has recently been challenged by the results of the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE),65 Interventional Management of Stroke (IMS III),66 and Intra-arterial Versus Systemic Thrombolysis for Acute Ischemic Stroke (SYNTHESIS EXP) trials.67 Mechanical thrombectomy devices received FDA approval for use in patients presenting up to 8 hours from symptom onset because of early recanalization being associated with a 4- to 5-fold improvement in clinical outcome.68 Further randomized, controlled trials are needed to test the clinical efficacy of new-generation stent-retriever (“stentriever”) thrombectomy devices.
Initiation of endovascular revascularization therapy provides targeted treatment at the site of the clot. Due to the associated risks of the procedure, if this is considered, more information for appropriate patient selection is needed to achieve an acceptable risk-benefit ratio.69,70 Poor response and poor outcomes with IV thrombolysis have been found with carotid terminus and large, proximal artery occlusions.71,72 Additionally, the outcome after endovascular therapy is also influenced by the composition and location of the thrombus, with improved recanalization rates for more proximal rather than distal thrombus.73⇓⇓⇓–77 Thus, there is some justification (level II) for vascular imaging of acute stroke patients at the time of the initial brain imaging study to triage patients to the best therapy and determine prognosis. This may also be the most practical and efficient time to obtain vascular imaging in stroke patients.
There are 3 major imaging strategies (and numerous combinations of these 3 strategies) used in acute ischemic stroke patients who are considered for endovascular revascularization therapy, with different underlying rationales (Fig 1). There is currently no definitive evidence supporting one strategy over the other. Some believe that more imaging provides additional, clinically relevant information, while others are concerned about the additional time resulting from the additional imaging and the potential delay to recanalization it might cause. The choice of imaging implemented may depend on physician preference and logistical factors (such as whether advanced imaging, especially MR imaging, can be performed quickly and on a 24/7 basis). In considering the underlying rationale for endovascular therapy, additional imaging may be more justified in patients within the 4.5- to 8-hour time window. In patients with a contraindication to IV tPA within the 0- to 4.5-hour time window and in patients considered for endovascular therapy after IV tPA failure, imaging the volume of the infarct may be sufficient.
The first strategy consists of going to the angiography suite immediately after the initial NCCT. The rationale for this approach is to minimize the door-to-recanalization time. In this setting, the vascular patency status is assessed on the DSA that precedes the therapeutic portion of the procedure, before lysis or removal of the clot. Collateral patterns can also be demonstrated, though infarct volume can only be indirectly assessed by attention to flow, parenchymal blush, and arterial-to-venous transit times. The second strategy consists of obtaining a CTA to assess vascular patency, with or without perfusion imaging, to better characterize the site of occlusion and the ischemic tissue before making an endovascular treatment decision. The third strategy consists of using MR imaging/MRA, possibly with diffusion- and perfusion-weighted imaging at institutions where it can be performed quickly and on a 24/7 basis. The rationale of these latter approaches is that the extra time needed to perform this additional imaging may be justified by the information gathered and the implications for decision-making.78,79 Some studies have demonstrated that the extra time for imaging until treatment does not adversely affect outcomes.80⇓–82
Imaging Evidence for Detection of Intravascular Clot
Vascular imaging of the acute stroke patient before endovascular therapy is necessary to determine whether an embolus/thrombus is present that is accessible and amenable to intra-arterial thrombolysis and/or mechanical thrombectomy. Imaging of the intracranial and extracranial vessels can be performed quickly and noninvasively by using CTA and MRA. However, DSA is considered the reference standard for detection of vascular stenoses and occlusions. CTA has been reported to have high sensitivity (97%–100%) and specificity (98%–100%) for detecting intracranial stenoses and occlusions compared with DSA (level Ib).83⇓⇓⇓⇓⇓⇓–90 MRA can also be used to characterize vascular patency (level Ib).4,64,91 CTA has been shown to be slightly superior to MRA for this purpose, typically for distal vascular lesions.83,84 Complete or partial signal void in regions of high and/or turbulent flow may occur on time-of-flight MRA, leading to an overestimation of stenosis. Window settings and presence of calcifications or adjacent bone can limit CTA evaluation.
CTA provides additional tissue information on the CTA-SI, initially thought to represent blood volume–weighted data. However, with current, faster CTA protocols, a steady-state is not always reached; the CTA-SI may be more blood flow–weighted and can frequently overestimate ischemic core relative to the DWI lesion volume.47 Hypoattenuated regions on CTA-SI indicate early ischemic changes that may be seen to better advantage compared with NCCT. In one study, the combined information from the CTA and CTA-SI demonstrated marked improvement in localization of both the ischemic core and the occluded vessel compared with NCCT and clinical information.92 Another advantage of CTA is that it can be obtained immediately following NCCT, after initiation of IV thrombolytic therapy in the CT scanner, to avoid delaying treatment.
Imaging Evidence for Detection of Viable Tissue
Determination of tissue viability based on imaging has the potential to individualize thrombolytic therapy and extend the therapeutic time window for some acute stroke patients. Although perfusion imaging has been incorporated into acute stroke imaging algorithms at some institutions, its clinical utility has not been proved. The potential value of perfusion imaging has been assessed in the Desmoteplase in Acute Ischemic Stroke–phase II (DIAS-II) trial by using MR diffusion/perfusion mismatch and a perfusion-CT mismatch as entry criteria to receive IV desmoteplase in patients presenting up to 9 hours from symptom onset.93 However, this trial failed to demonstrate superiority of treatment over placebo by using penumbral imaging as a selection criterion. Other trials such as Diffusion-weighted Imaging Evaluation For Understanding Stroke Evolution (DEFUSE), DEFUSE-2, and Echoplanar Imaging Thrombolysis Evaluation Trial (EPITHET) have shown promising results by using a combination of diffusion and perfusion imaging to identify good candidates for revascularization therapy beyond 3 hours.78,79,94 The MR RESCUE trial failed to demonstrate any difference in outcome in stroke patients selected by using penumbra imaging compared with no selection at all.65 Therefore, there is insufficient evidence at this point supporting the use of penumbra imaging to select patients for acute reperfusion therapy. Further randomized, controlled trials are needed to test the full spectrum of penumbra imaging selection for acute stroke therapies.
MR perfusion is employed at some institutions to assess the diffusion/perfusion mismatch. The presence of a perfusion abnormality larger than the DWI lesion (ie, a mismatch) is a qualitative marker for potential infarct expansion.95⇓–97 However, the extent of mismatched tissue varies greatly, depending on the perfusion parameter selected and the threshold selected to represent the PWI abnormality (level 2b).93,98⇓–100 Individual studies have reported varying perfusion parameters as most predictive of tissue viability and clinical outcome, without clear consensus. Some studies have suggested that the Tmax parameter (time-to-peak of the residue function) by using a threshold >6 seconds is a good predictor of infarct growth in the absence of early recanalization.101⇓–103
Perfusion CT is another method used to assess the ischemic core and penumbra. Similar to MR PWI, there is no clear consensus on the optimal perfusion parameter that is most predictive of tissue viability and outcome. A prospective multicenter study reported that an absolute cerebral blood volume threshold reflected the ischemic core and that a relative mean-transit-time threshold most accurately reflected the penumbra.104 However, in more recent and larger studies, relative cerebral blood flow was found to be more predictive of the ischemic core (nonviable tissue) than absolute CBV.105⇓⇓⇓⇓⇓⇓⇓–113 As for PWI, there is a need for standardization of the PCT methods used to define the ischemic core and the penumbra.
It is important to note that perfusion imaging has many applications beyond characterization of the penumbra and triage of patients to acute revascularization therapy. The negative results of the MR RESCUE trial do not negate these potential benefits.65 These applications include, but are not limited to, the following: 1) improving the sensitivity and accuracy of stroke diagnosis (in some cases, a lesion on PCT leads to more careful scrutiny and identification of a vascular occlusion that was not evident prospectively, particularly in the M2 and more distal MCA branches)46,113⇓–115; 2) excluding stroke mimics116; 3) better assessment of the ischemic core114 and collateral flow117; and 4) prediction of hemorrhagic transformation and malignant edema.118,119
Imaging Evidence for the Characterization of Collateral Vessels
The concept of collaterals as a vascular network that can potentially bypass devastating effects of a blocked cerebral artery has recently gained momentum. Collaterals have been shown to enhance recanalization and reperfusion, reduce the size of the core and ischemic lesion growth, decrease the risk of hemorrhagic transformation, and improve outcomes with IV and endovascular revascularization (level III).117,118 More specifically, a poor collateral pattern has a high specificity for poor tissue and clinical outcome (level III).120
Several imaging approaches have been proposed to evaluate collaterals, including CTA, PCT, perfusion-weighted imaging, DSA, arterial spin-labeling (ASL), and positron-emission tomography. Currently, none of these techniques is absolute nor is any established as a reference standard to assess and quantify collateral flow. Imaging techniques that include a serial, temporal assessment have a definitive advantage because of the dynamic nature of collateral perfusion. Optimized imaging analyses of collateral perfusion patterns may have to consider the underlying mechanism of arterial occlusion, as patterns may vary from intracranial atherosclerosis to cardioembolism.121 Thresholded volumes of hypoperfusion on perfusion maps may not be as informative as voxel-based measures that depict the heterogeneity of the penumbra.122
Summary.
In acute stroke patients who are candidates for endovascular therapy, vascular imaging (CTA, MRA, DSA) is strongly recommended during the initial imaging evaluation.4,57,63,64 Perfusion imaging may be considered to assess the target tissue “at risk” for reperfusion therapy.4,64 However, the accuracy and usefulness of perfusion imaging to identify and differentiate viable tissue have not been well-established.
Acute large-vessel intracranial thrombus is accurately detected by CTA, MRA, and DSA.
Patients with large infarctions tend to have poor outcomes. The ischemic core is determined most accurately with DWI. Appropriately thresholded PCT-CBV and PCT-CBF can also be used to identify the ischemic core despite immediate reperfusion.
A poor collateral pattern has a high specificity for poor tissue and clinical outcome (level III).
Rationale and Imaging Evidence for Acute Ischemic Stroke Patients Who Are NOT Candidates for IV or Endovascular Therapy and Patients with Transient Ischemic Attacks
When acute revascularization therapy is not being considered, the role of imaging is primarily focused on diagnosis, prevention of immediate complications, and the identification of potentially treatable causes of future stroke. In patients with TIAs, multimodal MR imaging is preferred, and NCCT should be obtained only if MR imaging is not available, as NCCT has limited utility in patients whose symptoms have resolved.123 DWI can demonstrate lesions in approximately 40% of TIA patients,56,124,125 and DWI positivity in TIA patients is associated with a higher risk of recurrent ischemic events.126 The distribution of the DWI lesions can help with the determination of the stroke etiology (scattered emboli in multiple territories indicative of proximal embolic source [eg, cardiac], watershed distribution of lesions suggestive of carotid disease, and so forth).127⇓–129
MR-based perfusion imaging, either with dynamic susceptibility contrast or ASL, may additionally identify a vascular etiology in TIA patients.130,131
CTA or MRA of the intracranial and cervical arteries and duplex sonography (DUS) for the cervical arteries are used to identify stenosis and/or occlusion (level Ib)123 and determine appropriate secondary prevention, such as extracranial carotid revascularization, for these patients. An appropriate evaluation for cardiac sources of TIA/stroke (eg, echocardiography) should also be performed.
Summary.
When revascularization therapy is not indicated or available, multimodal neuroimaging of the brain and cerebrovasculature with MR imaging should be performed to confirm the diagnosis of stroke, identify the underlying etiology, and assess immediate complications and risk of future stroke.123
Multimodal CT, including NCCT and CTA and possibly PCT, should be reserved for patients who have contraindications to MR imaging, or if MR imaging is not available.123
Rationale and Imaging Evidence for Acute Ischemic Stroke Patients with Wake-Up Stroke or More Generally with Unknown Time of Onset
Acute stroke patients presenting without a definite time of symptom onset, such as wake-up stroke, may or may not proceed to thrombolytic treatment. If no acute reperfusion therapy is considered, NCCT is recommended to assess for intracranial hemorrhage. Further imaging evaluation is consistent with recommendations discussed in the previous sections.
However, if acute reperfusion therapy is considered, typically as part of a clinical trial, multimodal MR imaging (by using the DWI-PWI mismatch or the DWI-FLAIR mismatch) or multimodal CT (NCCT, CTA, and PCT) is required to assess the “tissue clock,” as the time clock concept does not apply.132⇓–134
Summary.
In acute stroke patients without a definite time of symptom onset, imaging recommendations depend on whether acute reperfusion therapy may be performed.
If no acute reperfusion therapy will be performed, imaging recommendations are consistent with those in the previous sections.
If acute reperfusion therapy is considered, multimodal MR imaging or CT with perfusion imaging is recommended to evaluate viable tissue, as the time clock is not applicable. However, there is no firm evidence supporting imaging selection for treatment in this patient population.
Rationale and Imaging Evidence for Patients Suspected of Posterior Fossa Stroke
Acute stroke imaging in patients presenting with posterior fossa infarctions is quite similar to hemispheric ischemic stroke. A few aspects specific to the posterior fossa include the following:
NCCT is relatively insensitive in detecting acute and small cortical or subcortical infarctions, especially in the posterior fossa. PCT has very limited indications for the posterior fossa as beam-hardening artifacts from the temporal bones limit the image quality. Additionally, the spatial resolution of PCT is challenged by the small size of ischemic lesions in the posterior fossa. Of further consideration, PCT imaging of the posterior fossa may involve inclusion of the ocular lenses in the cine imaging acquisition, which is associated with a non-negligible deterministic risk of cataract formation.
MR imaging with DWI is the optimal imaging technique to assess for ischemic lesions in the posterior fossa (level Ia).130 It can assess the degree of brain stem infarction before intra-arterial treatment. However, due to the dismal prognosis of basilar occlusion, a higher risk is often tolerated to achieve recanalization at any time point.
CTA, MRA, and DSA are the preferred imaging techniques to assess for basilar artery thrombosis (level Ia).
Summary.
In acute stroke patients presenting with posterior fossa infarction, imaging recommendations are similar to hemispheric acute ischemic stroke.
MR imaging with DWI is the optimal imaging technique to assess the presence and extent of ischemia in the posterior fossa.
CTA and DSA are the preferred imaging techniques to assess for basilar artery thrombosis. MRA is an acceptable alternative for patients already undergoing an MR imaging examination.
Rationale and Evidence Supporting Imaging of the Cervical Arteries in Acute Stroke and TIA Patients
Imaging of the cervical arteries (in addition to imaging of the intracranial arteries) should be performed routinely as part of the imaging evaluation of patients with acute ischemic stroke but should not delay IV tPA administration in the first 4.5 hours.4,64 Similarly, noninvasive imaging of the cervical arteries should be a routine component of the imaging work-up of patients with TIAs.4,64 The primary goal of imaging the cervical arteries is to help identify the mechanism of the stroke and thus potentially to prevent a recurrence.4,64,135 Several imaging techniques are available to assess the cervical arteries including DUS, CTA, MRA, and DSA.136⇓–138 Each technique has its own advantages and limitations in specific clinical situations, but overall, these noninvasive techniques show general agreement with DSA in approximately 90% of cases (level Ib).139⇓–141 DSA is considered the reference standard imaging technique to assess the degree of stenosis and determine patient eligibility for carotid endarterectomy/angioplasty/stent placement. The concordant results of 2 noninvasive techniques (DUS, CTA, and/or MRA) can be used to determine treatment eligibility, avoiding catheterization risks.142,143 A 99% stenosis (the so-called string sign) is most accurately detected by DSA, followed closely by CTA and contrast-enhanced MRA.144
Summary.
In acute stroke patients, vascular imaging should be performed to evaluate the mechanism of stroke and assess risk of future stroke.1
Overall, vascular imaging with DUS, CTA, MRA, or DSA has good agreement.
Concordant results from at least 2 noninvasive imaging techniques can be used to determine treatment eligibility for revascularization procedures.
Acknowledgments
We would like to thank Judy Burleson, MHSA, Director, Metrics, American College of Radiology and Christine Waldrip, RN, MHA, Program Manager, American College of Radiology Appropriateness Criteria, for the support they provided in the preparation of this manuscript.
Footnotes
M. Wintermark and P.C. Sanelli are co-first authors of this article.
A shortened version of this article is published in the Journal of the American College of Radiology.4
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 3a.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.↵
- 144.↵
- © 2013 by American Journal of Neuroradiology