Abstract
SUMMARY Evolving techniques in interventional neuroradiology have widened therapeutic options, allowing treatment even in complex cases. Complex neuroendovascular procedures (eg, stent-assisted coiling, stent placement in X- or Y-techniques) require precise delineation of cerebral vasculature and devices. However, because of the complex anatomy or if an ideal projection is not possible, visualization of the parent artery might be difficult. We present 2 complex cases of basilar tip aneurysm in which ACT proved to be beneficial in the intraprocedural monitoring of stent-assisted coil embolization.
ABBREVIATIONS:
- ACT
- angiographic CT
- GDC
- Guglielmi detachable coil
- PCA
- posterior cerebral artery
Evolving techniques in interventional neuroradiology have widened therapeutic options. Therefore, interventional neuroradiologists also treat cases that, to some extent, necessitate a combination of methods (eg, stent-assisted coiling, stent placement in X- or Y-techniques1⇓–3). These complex neuroendovascular procedures require precise delineation of cerebral vasculature and devices. However, because of complex anatomy or if an ideal projection is not possible, visualization of the parent artery might be difficult. ACT uses rotational C-arm flat panel detector technology capable of high-spatial-resolution volumetric imaging. Within the angiosuite, ACT allows immediate detection or exclusion of peri- or postprocedural complications such as intracranial hemorrhage, for example.4,5 In addition, ACT has proved to be a reliable technique in neuroendovascular procedures such as stent-assisted coiling.6 Here we present 2 cases of basilar tip aneurysms in which ACT proved to be beneficial in the intraprocedural monitoring of stent-assisted coil embolization.
Technique
ACT imaging was performed on a biplane flat panel detector angiographic system (Axiom Artis dBA; Siemens Healthcare, Erlangen, Germany). ACT acquisition was obtained by using standard parameters provided by commercially available software (syngo XWP, DynaCT, InSpace 3D software; Siemens), which have been described in a previous article7: briefly, 20-second rotation; 0.4° increment; 1240 × 960 matrix in projections at zoom 0 after resampling; 200° total angle; system dose, 1.2 μGy/frame. Images were automatically corrected for gain of the image intensifier during the acquisition. There is no contrast medium required for this technique.
Postprocessing consisted of computed correction of beam-hardening, ring artifacts, and scattered radiation (ACT-preset). The selectable postprocessing algorithm included setting “bone-kernel” and “hard” image characteristics. Secondary calculations of image data were performed with a volume of approximately 7.5 × 7.5 × 7.5 cm and a 512 × 512 matrix, with a resulting isotropic voxel size of approximately 0.15 mm in each plane. Multiplanar reconstructions and maximum intensity projections in axial and sagittal orientations to the stent and coil package were obtained for better visualization of stent or arterial patency or potential migration of coil material. Overall, ACT acquisition requires only 2–3 minutes (40 seconds for positioning and rotation, 1–2 minutes for postprocessing) and could be displayed simultaneously within the angiosuite, rendering patient transfer to CT unnecessary.
Case 1
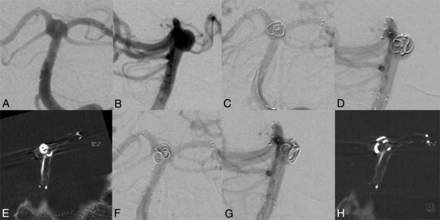
A 56-year-old women was scheduled for coil embolization of an incidental wide-neck basilar tip aneurysm (6 × 10 mm), incorporating both P1 segments into the aneurysm (Fig 1A, -B). Before the coiling, a stent (Neuroform3 Microdelivery Stent, 4.0 × 20 mm; Boston Scientific, Natick, Massachusetts) was deployed in the right PCA and the basilar artery. Subsequently, a second stent (Solitaire AB, 4.0 × 20 mm; ev3, Irvine, California) was passed through the stent struts of the Neuroform stent. The distal end of the stent was positioned in the left PCA, causing a Y-configuration and therefore recreating an aneurysm neck. After we positioned a microcatheter through the stent struts into the aneurysm, a 3D coil was inserted (Cashmere Microcoil, 4 × 45; Micrus Endovascular, San Jose, California). Because stents are, with the exception of proximal and distal radiopaque markers, hardly visible in standard techniques such as DSA, even with multiple oblique projections, it was not possible to ascertain coil deployment exclusively within the aneurysm (Fig 1C, -D). Thus, ACT was performed to accurately evaluate coil position before detachment, revealing a displaced coil loop in the P1 segment of the left PCA (Fig 1E). Repeat ACT after repositioning confirmed the correct coil position in the basilar tip aneurysm without compromising a parent artery (Fig 1F - H). Detachment of subsequent coils resulted in complete occlusion of the aneurysm (not shown).
A and B, Basilar tip aneurysm before coiling. C and D, The first coil in a basilar tip aneurysm before detachment. E, ACT scan shows coil protrusion into the left P1 segment. F and G, Delineation of the coil after repositioning without evidence suggesting coil protrusion in ACT (H).
Case 2
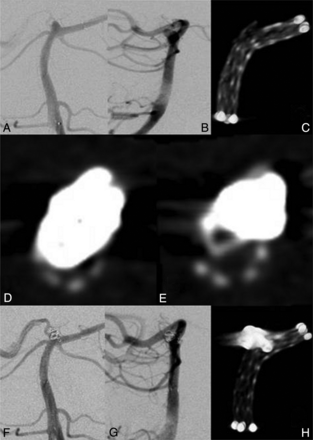
A 66-year-old man presented with an incidental wide-neck basilar tip aneurysm (2 × 3 mm) incorporating the left P1 segment (Fig 2A, -B). A stent (Neuroform3, 3.5 × 20 mm; Boston Scientific) was placed in the left PCA and the basilar artery. Because of the curved position, there was an opening of the stent struts at the sharpest angle (Fig 2C). Then, a microcatheter was passed through the stent struts into the aneurysm, and 2 coils (GDC-US, 2.5 × 30 mm, GDC-US, 2 × 20 mm; Boston Scientific) were inserted. Because there was still perfusion of the aneurysm, a third coil (GDC-US 2 × 10 mm; Boston Scientific) was inserted. Before detachment, DSA was suggestive of coil protrusion into the left P1 segment. ACT confirmed coil protrusion, most likely through the opened stent struts, resulting in an approximately 80% stenosis of the stent (Fig 2D). After multiple attempts at coil repositioning, there was still a small protrusion through the stent struts into the left P1 segment. Compared to previous coil positions, however, there was only a 25%–30% narrowing of the parent artery detectable (Fig 2E). Because additional attempts at coil repositioning or removal of the third coil were considered a disproportionate risk for the patient, detaching the coil in the current position (Fig 2F, -H) was preferred. To our knowledge, there were no neurologic symptoms present after the intervention or at regular follow-up visits within 2 years.
A and B, A 2 × 3 mm basilar tip aneurysm. C, ACT of the stent showing the opening of the stent struts. D, ACT image axial to the stent showing a profound narrowing of the stent. E, After repositioning, there is less protrusion of the coil loop, resulting in a decreased stenosis of the stent. F–H, Final imaging after coiling.
Discussion
Here we presented 2 cases of wide-neck basilar tip aneurysms incorporating 1 or both P1 segments, thus needing stent-assisted coiling. Because of the complex anatomy, especially for the first case, visualization of the parent artery or the stent was not feasible even with multiple oblique projections and DSA could not sufficiently exclude coil protrusion. Intraprocedural ACT clearly showed coil protrusion compromising arterial patency before detachment, which could be corrected, and it ascertained complete or almost complete arterial patency after coil repositioning.
Although ACT is also an established technique to identify pre-, peri-, or postprocedural complications like hemorrhage or hydrocephalus,5,8 in our cases, ACT was primarily performed for multiplanar visualization of endovascular stents and coil material.6 Beam-hardening artifacts caused by the coil package impair visibility of the stent. As demonstrated in Figs 1E and 2D, -E, beam-hardening artifacts impair visibility right after the first coil is inserted and increase with consecutively inserted coils. This problem limits the usefulness of ACT in cases with aneurysm (coil package) size mainly >10 mm in diameter but allows an excellent or at least favorable overview of the stent with adjacent small coil packages of ≤10 mm in diameter.6 When DSA cannot reliably delineate the coil position, intraprocedural ACT could help to identify even small or “silent” protruded coil loops in small coil packages.
Regarding radiation dose, preliminary results from internal investigations (T.S., 2011, unpublished data) with a calibrated ionization chamber and thermoluminescence dosimetry as well as Monte Carlo simulations and virtual phantom models show the effective dose of an ACT at a level of approximately 1.5 mSv. With collimation, this dose could be further reduced. Overall, we consider this as an acceptable dose to achieve a significant diagnostic benefit in the setting of this disease. Thus, ACT may provide the best positioning of coil material in a timely suitable manner and, compared with multiple oblique projections by using DSA, without the necessity of contrast medium.
Conclusions
Intraprocedural ACT might be helpful to accurately determine coil position during complex neuroendovascular procedures when it is difficult to visualize vasculature in DSA. Thus, intraprocedural visualization of vessel patency as well as coils and stents by using ACT may reduce procedural complications and increase safety.
REFERENCES
- Received October 31, 2011.
- Accepted after revision December 21, 2011.
- © 2013 by American Journal of Neuroradiology