Abstract
BACKGROUND AND PURPOSE: Asymptomatic carotid stenosis of ≥70% increases the incidence of microembolism and/or chronic hypoperfusion, which may consequently impair neurocognition and brain connections. We sought controlled evidence for any cognitive benefit of aggressive medical therapy and combined carotid revascularization.
MATERIALS AND METHODS: Patients with asymptomatic, unilateral, ≧70% stenosis of the extracranial ICA chose either aggressive medical therapy alone or in combination with carotid artery stent placement in this nonrandomized controlled study. They were examined with a battery of neuropsychological tests, structural MR imaging, DTI, and resting-state fMRI before and 3 months after treatment.
RESULTS: Forty patients were included with 15 in the medical group and 25 in the stent-placement group. Among them, 13 and 21 in the respective groups completed neuroimaging follow-up. The baseline characteristics and the changes in cognitive performance during 3 months showed no differences between treatment groups. Nevertheless, compared with the medical group, the stent-placement group showed subjective dizziness alleviation (P = .045) and a small increase in fractional anisotropy at the splenium of the corpus callosum and the posterior periventricular white matter ipsilateral to carotid artery stent placement. Moreover, only the stent-placement group showed interval improvement in immediate memory and visuospatial performance, which was accompanied by an increase of functional connectivity at the insular cortex of the dorsal attention network and the medial prefrontal cortex of the default mode network.
CONCLUSIONS: Both aggressive medical therapy alone and combined carotid revascularization in ≧70% asymptomatic carotid stenosis similarly preserved cognition during 3-month follow-up, though the latter had the potential for dizziness alleviation and cognitive and connectivity enhancement.
ABBREVIATIONS:
- CAS
- carotid artery stent placement
- FA
- fractional anisotropy
- Fc
- functional connectivity
- MCI
- mild cognitive impairment
- VCIND
- vascular cognitive impairment no dementia
Interventional revascularization for ≥60% asymptomatic ICA stenosis has long been debated, given the decreasing annual risk of ipsilateral ischemic stroke in these patients from 2.3% to 0.5% with the development of contemporary optimal medical treatment.1⇓⇓–4 However, some of these patients carry a higher risk of stroke than others despite optimal medical treatment. Patients with detectable embolic signals by transcranial Doppler have a high annual risk (7%) of stroke.5 Stenotic degree of ≥90%, poor collaterals, and echolucent plaque texture could also stratify patients into groups with varying high stroke risk to >4% annually.6,7 Thus, interventional revascularization should be considered in such patients. Recently, long-term randomized trials, the Asymptomatic Carotid Trial8 and the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST)9 demonstrated that there was no difference in the rate of late ipsilateral stroke after carotid endarterectomy or carotid artery stent placement (CAS) in asymptomatic and symptomatic patients. Of asymptomatic patients, the 5-year cumulative rate of stroke-free survival was 93.1% in the CAS group and 94.7% in the carotid endarterectomy group.8
Hence, asymptomatic carotid stenosis has been viewed from a changing perspective from stroke risk to cognitive susceptibility.10,11 We previously demonstrated that patients with unilateral asymptomatic carotid stenosis of ≥70% had more dizziness/unsteadiness and poorer verbal memory, executive function, and visuospatial perception than the healthy controls, accompanied by extensive widespread disruption of long-range structural and functional connectivity.12,13 The mechanisms are likely attributed to microemboli from unstable carotid plaques5 and/or chronic hypoperfusion.14,15 Single-arm studies of carotid revascularization accomplished by either carotid endarterectomy or CAS reported the controversial results of cognitive enhancement in patients with asymptomatic carotid stenosis.15⇓⇓⇓⇓–20 However, there is a lack of medical-controlled evidence reflecting contemporary medical improvement and risk-benefit balance of interventions for cognitive preservation. Here, we investigate the impact of aggressive medical treatment with or without combined carotid revascularization on neurocognitive and connectivity outcomes at 3 months after treatment in patients with ≧70% asymptomatic carotid stenosis.
Materials and Methods
Subjects, Treatment, and Neuropsychological Tests
We enrolled patients with asymptomatic, unilateral severe stenosis of the extracranial ICA at our dizziness outpatient clinic of Taipei Veterans General Hospital between March 2010 and July 2015. The inclusion criteria were between 20 and 80 years of age and ICA stenotic degree of ≥70% identified by both duplex ultrasonography21 and gadolinium-enhanced MR angiography (North American Symptomatic Carotid Endarterectomy trial criteria).22 The exclusion criteria included transient ischemic attack or stroke, functional disability (modified Rankin Scale score of ≥3), carotid dissection, and the presence of contralateral ICA stenosis of ≥50% and comorbidities of dementia, major depression (based on the Diagnostic and Statistical Manual of Mental Disorders-IV), Parkinsonism, multiple sclerosis, brain tumor, congestive heart failure (left ventricular ejection fraction <40%), chronic obstructive pulmonary disease, cirrhosis, renal failure (estimated glomerular filtration rate <30 mL/min/1.73 m2), and malignancy. The medications of all subjects were recorded. Written informed consent was obtained from each participant before enrollment. This study was approved by the ethics committee of the Taipei Veterans General Hospital (VGHIRB No. 2012–01-016AC).
All patients received aggressive medical treatment (dual antiplatelets if tolerated or at least 1 antiplatelet, statin therapy goal of low-density lipoprotein of <100 mg/dL, diabetes treatment goal of glycated hemoglobin level of <7%, hypertension treatment goal of systolic blood pressure of <140 mm Hg, smoking cessation) with or without carotid revascularization treatment in a nonrandomized fashion tailored for the individual procedure and preference. For CAS, conventional angiography of the supra-aortic arteries and branches was performed by using a transfemoral arterial approach. An embolic protection device (FilterWire EX or EZ; Boston Scientific, Natick, Massachusetts) was carefully navigated through the stenotic lesion and placed in the distal cervical ICA. Then a self-expandable stent (Wallstent, Boston Scientific; or Precise; Cordis, Fremont, California) was introduced and adjusted to the dimension of the stenotic artery, followed by postdilation with a balloon of 5–6 mm in diameter. Angiography was repeated for the ICA and its intracranial branches to ensure the residual stenosis of the target site was <50% and absence of endovascular complications.
All subjects were evaluated with a battery of neuropsychological tests before and 3 months after treatment by a blinded trained examiner, including the Dizziness Handicap Inventory,23 the Mini-Mental State Examination, memory tests (verbal selective reminding test; an auditory verbal learning test, including total immediate recall and 15-minute delayed recall of 12 items),24 executive tests (the Modified Trail-Making Test A and B25; the Stroop Color and Word Test26), an attention test (the Symbol Digit modalities Test),27 and complex visuospatial perception tests (the Modified Complex Figure Test with Copy and Recall).
MR Imaging Acquisition
Before and 3 months after the treatment, patients were subjected to MR imaging and instructed to hold still, keep their eyes open, and think of nothing in a 3.0 Discovery 750 (GE Healthcare, Milwaukee, Wisconsin) MR imaging scanner. All images were acquired along the anteroposterior commissural plane, according to multiplanar T1-weighted BRAVO anatomica images (http://www3.gehealthcare.com/en/Products/Categories/Magnetic_Resonance_Imaging/Neuro_Imaging/BRAVO) (TR = 12.2 ms; TE = 5.2 ms; flip angle = 12°; voxel size = 1 × 1 × 1 mm; FOV = 256 × 256 mm). A series of fluid-attenuated inversion recovery sequences was acquired to rate leukoaraiosis severity. The stent-placement group received additional diffusion-weighted imaging and apparent diffusion coefficient imaging within 3 days after the procedure to exclude any periprocedural insult. For DTI, a single-shot diffusion spin-echo echo-planar imaging sequence (TR/TE = 9500/85.6 ms; thickness = 2 mm; matrix = 128 × 128; FOV = 256 × 256 mm; 30 directions) was adopted. For resting-state fMRI, the blood oxygen level–dependent signals from a task-free run (124 time points/372 seconds) of a gradient-echo echo-planar imaging sequence (TR/TE = 3000/30 ms; flip angle = 90°; FOV = 222 × 222 mm; thickness = 3 mm) were recorded.
MR Imaging Processing and Analysis
A blinded neurologist and a neuroradiologist reviewed all images. The severity of leukoaraiosis was assessed by the semiquantitative Scheltens rating scale.28 The hemisphere ipsilateral to the ICA stenosis was flipped to the right side along the midsagittal plane. We analyzed T1-weighted anatomic images and manually outlined the bilateral hippocampi to calculate the hippocampal volumes of each patient29,30 and brain volume by using the voxel-based morphometry approach.31 Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm12) was used to segment the gray and white matter intensities and normalize them to Montreal Neurological Institute space. The gray and white matter volumes were compared within each group by paired t tests with a threshold of P < .05. For DTI, voxelwise fractional anisotropy (FA) was analyzed after applying preprocessing with Tract-Based Spatial Statistics from the FMRIB Software Library (TBSS; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS), as previously described.12 We performed a paired t test for within-group interval changes of the FA maps and then a 2-sample t test for between-group comparison of the interval changes with a significance set at P < .05 with family-wise error rate correction for multiple comparisons (random permutations, n = 5000).
The mean FA values of the whole brain or each hemisphere, as well as of the focal clusters with significant interval changes, were extracted in each patient for statistical analysis. For resting-state-fMRI, preprocessing and analytic procedures were performed as previously described.13 ROIs with 4-mm radii were defined in the hemisphere ipsilateral to ICA stenosis (flipped to the right), representing the seed regions for 6 resting-state networks, including the posterior cingulate cortex (0, −50, 22) and the medial prefrontal cortex (1, 48, −4) for the default mode network, right frontal eye field (26, 6, 48) for the dorsal attention network, the middle frontal gyrus (45, 29, 32) for the frontoparietal network, the primary motor cortex (41, −20, 62) for the sensorimotor network, the dorsal anterior cingulate cortex (1, 10, 46) for the salience network, and, last, the primary visual cortex (4, 81, −10) for the visual network as a control supplied by the vertebrobasilar circulation.13
The temporal correlations between the blood oxygen level–dependent signals from each ROI and brain-wise voxels were calculated and presented as Pearson correlation coefficients (r), followed by a Fisher r-z transformation. Z values from a single ROI in each network were defined as functional connectivity (Fc) and were computed with 1-sample t tests by using SPM8 to generate the Fc map in both groups. For within-group analysis, the Fc interval changes in each group were obtained by a paired t test, followed by false discovery rate correction with a significance defined as q < .05.
Statistical Analyses of Demographic/Neuropsychological Variables and Multivariate Regression Models
SPSS software (Version 18.0; IBM, Armonk, New York) was used for the statistical analyses. Categoric variables between groups were compared by using χ2 or Fisher exact tests if the expected number was ≤5. The baseline dizziness, neuropsychological tests, leukoaraiosis scores, hippocampal volumes, and the mean FA values were compared by 2-sample t tests between groups. The within-group interval changes of parameters were compared by paired t tests. The between-group interval changes of each value were then compared by using 2-sample t tests.
Significance was defined as P < .05. The significance of 9 neuropsychological measures was corrected by the Bonferroni method (P < .0056). The changes of the dizziness scale and neuropsychological scores were classified as improvement from the baseline (>0), no change (= 0), or decline (<0), and the percentages of each condition were compared between groups by using χ2 or Fisher exact tests. To investigate the relationship between the connectivity measures (ie, FA or Fc) and the neuropsychological changes after treatments, we used a multivariate regression model adjusted for age, sex, years of education, treatment group, the stenotic degree, the baseline presence of mild cognitive impairment (MCI), and vascular risk factors. We defined MCI or vascular cognitive impairment no dementia (MCI/VCIND) with a delayed verbal recall score of <8 (ie, 1.5 SDs below the mean of the healthy controls according to the previous literature).13,32
Results
Ischemic Events and the Neurocognitive Changes after Treatment
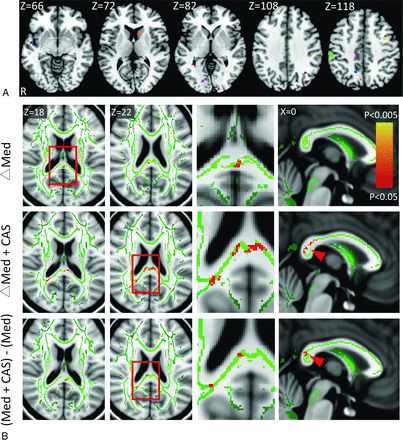
Forty-seven subjects were consecutively enrolled, with 5 being excluded due to the presence of ≥50% stenosis in the bilateral ICA and 2 being lost to follow-up. Therefore, 40 subjects, 15 in the medical group and 25 in the stent-placement group, completed the neuropsychological follow-up; 34 of them also completed the neuroimaging follow-up (13 and 21 in respective groups). The baseline characteristics, scores on the Dizziness Handicap Inventory and neuropsychological tests, percentage of MCI/VCIND, Scheltens leukoaraiosis score, hippocampal volumes, and hemispheric mean FA values between groups were not different (Table 1). Six of 15 patients in the medical group (40%) and 7 of 25 in the stent-placement group (28%) were considered to have MCI/VCIND (P = .318). The stent-placement group had 100% successful carotid revascularization with residual stenosis of <50% and no periprocedural events, though 12 patients (48%) had asymptomatic tiny cerebral emboli after the procedure according to MR imaging (Fig 1A).
Baseline characteristics
A, Procedure-related microemboli based on the diffusion-weighted images are overlaid on a standard Montreal Neurological Institute template from 12 of 25 patients in the stent-placement group, indicated by different colors. B, The increases (red-yellow) of fractional anisotropy (the white matter skeleton is shown in green) at 3 months after aggressive medical therapy alone (Med, upper row) or combined carotid artery stent placement (Med+CAS, middle row) and the between-group comparisons (lower row). The carotid stenotic side was set to the right in all subjects. The third column from the left represents the high-power views of the insets. Note significant FA increases at the posterior corpus callosum (arrowheads) and the posterior periventricular white matter ipsilateral to the CAS in the stent-placement group.
At 3 months after treatment, there were no vascular events among all subjects. There was no between-group difference in the changes of neurocognitive function, except that the stent-placement group showed subjectively better dizziness alleviation (P = .045) compared with the medical group. However, the stent-placement group, but not the medical group, had notable within-group improvement in the total immediate recall of verbal memory (P = .001, uncorrected; with P < .0056 as significant) and the visuospatial performance (Complex Figure Test [Copy], P = .001, uncorrected) (Table 2). In the total immediate recall test, 21 of 25 in the stent-placement group (84%) and 9 of 15 in the medical group (60%) showed improvement after treatment (P = .057), while 3 in the stent-placement group (12%) and 3 in the medical group (20%) performed worse (P = .199).
Interval changes within and between groups
Changes of Structural and Functional Connectivity by Treatment
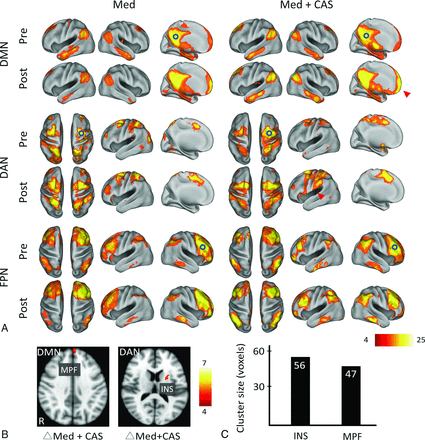
Most interesting, both groups showed localized FA increases at the posterior corpus callosum after treatment. The between-group comparison of the FA changes showed that the stent-placement group had small-but-significant FA increments at the posterior corpus callosum and the posterior periventricular white matter ipsilateral to the stenosis/CAS compared with the medical group (Fig 1B). Neither the leukoaraiosis score nor the hemispheric mean FA showed notable changes in both groups (Table 2). On the examined functional networks, we noted within-group, but no between-group, enhancement of Fc strength in the stent-placement group, but not in the medical group, between the posterior cingulate cortex and the medial prefrontal cortex contralateral to the stenosis/CAS in the default mode network as well as between the frontal eye field and the insular cortex contralateral to the stenosis/CAS in the dorsal attention network (Fig 2A, -B).
A and B, The functional connectivity correlation maps of both groups (Med indicates medical group; Med+CAS, stent placement group) before (pre) and 3 months after treatment (post). The carotid stenotic side was set to the right. Hollow circles indicate the predefined ROIs for individual networks at the right brain. Color bars represent T values. Q indicates the false discovery rate–corrected P value. The stent-placement group, not the medical group, showed within-group enhancement of Fc at the medial prefrontal cortex (MPF, T = 5.27, cluster size = 47, Q = .027) of the default mode network (DMN) and at the insular cortex (INS; T = 5.35, cluster size = 56, Q = .040) of the dorsal attention network (DAN) (arrowheads). C, The bar chart of the aforementioned cluster sizes with increased Fc is shown. FPN indicates frontoparietal network.
Correlation between Neurocognitive Changes and Connectivity Measures
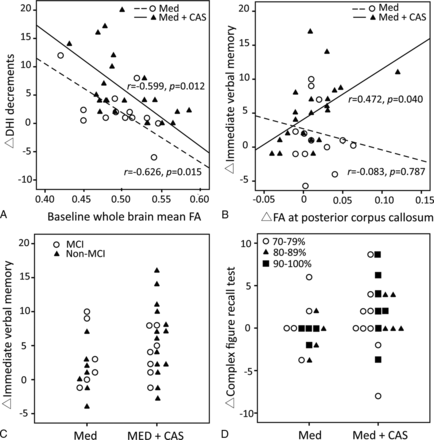
Using a multivariate regression model, we found that the baseline whole-brain mean FA (P = .002) and the treatment technique (stent-placement better, P = .034) correlated with the decreases in dizziness (Dizziness Handicap Inventory) (R2 = 0.411) after adjusting for age, sex, years of education, stenotic degree, presence of MCI, and vascular risk factors. Figure 3A shows a reverse linear relationship between the whole-brain mean FA and decreases in the Dizziness Handicap Inventory in both groups, suggesting the lower mean connectivity of the patients at baseline and more dizziness alleviation felt after treatment. With regard to the variables affecting the total immediate recall scores, age (P = .021) and interval changes of focal FA at the posterior corpus callosum (P = .040) correlated with the changes of the total immediate recall performance (R2 = 0.331) in the stent-placement group, but not in the medical group (Fig 3B). Neither the baseline status of MCI/VCIND (Fig 3C) nor the baseline stenotic degree predicted the changes of either total immediate recall scores or complex figure recall scores (Fig 3D).
Scatterplots of the correlation analyses in the medical (Med) and the stent placement group (Med+CAS). A, The baseline whole-brain mean fractional anisotropy negatively correlates with dizziness alleviation (decreases in Dizziness Handicap Inventory [DHI]) in both groups. B, The focal FA increases in the posterior corpus callosum positively correlate with the improvement of immediate verbal memory only in the stent-placement group. C, The relationship is shown between the baseline presence of mild cognitive impairment/vascular cognitive impairment no dementia and the improvement of immediate verbal memory in the 2 groups. D, The baseline stenotic degree is not related to the changes of Complex Figure Test (Recall) scores.
Discussion
This was a nonrandomized controlled study of revascularization plus aggressive medical therapy for severe asymptomatic carotid stenosis with respect to the possibility of cognitive and connectivity enhancement. We found that combined revascularization and aggressive medical treatment significantly alleviated subjective dizziness but did not enhance cognitive performance after 3 months compared with the aggressive medical treatments alone. The above findings were accompanied by greater increases of microstructural connectivity at the splenium of the corpus callosum and the posterior periventricular white matter ipsilateral to the stenosis/CAS. The baseline whole-brain mean FA was inversely correlated with the dizziness alleviation. Moreover, only the stent-placement group showed interval improvement in the short-term verbal memory and visuospatial performance after 3 months. Most interesting, the higher the FA increase at the posterior corpus callosum after CAS, the greater was the improvement in short-term verbal memory, suggesting that augmented microstructural connectivity of the posterior white matter might mediate revascularization-related cognitive changes. The stent-placement group also had focal increases of Fc at the medial prefrontal cortex in the default mode network and at the insula in the dorsal attention network contralateral to the stenosis/CAS, which we previously disclosed as susceptible regions in unilateral severe asymptomatic carotid stenosis patients.13 These Fc changes were not significantly different between groups but might implicate partial reversibility by a combined revascularization therapy. Thus, it is important to identify those asymptomatic patients at risk and offer timely treatment.
A previous uncontrolled case series of uncomplicated carotid endarterectomy in symptomatic (n = 50) and asymptomatic (n = 30) patients with >70% carotid stenosis showed an increase of the hemispheric mean FA ipsilateral to the surgery site after 1 month in association with posttreatment cognitive improvement.33 In contrast, others reported postoperative memory decline in a portion of patients with symptomatic or asymptomatic carotid stenosis 1 month after undergoing carotid endarterectomy or CAS. The multivariate regression analysis showed that memory decline was associated with periprocedural microemboli (11/21 = 52%) and baseline neurologic deficits.34 In our study, a similar proportion (48%) in the stent-placement group was found to have procedure-related silent microemboli. Nevertheless, we found a modest memory enhancement instead of decline in the stent-placement group and no correlation between the microemboli and cognitive changes at 3 months. The focal FA at the watershed posterior corpus callosum and the posterior periventricular region, rather than the hemispheric mean FA, increased, particularly in the stent-placement group. The posterior corpus callosum (ie, the splenium) is supplied by both the anterior cerebral artery and the posterior cerebral artery,35 perfusion of which can be augmented by revascularization therapy. The nearby retrosplenial cortex is structurally connected with the medial prefrontal cortex and medial temporal regions and involved in memory processing36 with the precuneus, posterior cingulate cortex, and hippocampus.37 Lesions in the splenium or the retrosplenial cortex have been reported to result in verbal and visual memory deficits.38,39 The cellular components of the observed FA or Fc increases are still unknown. They can be attributed to increased vasodilation and blood flow, improved neurovascular reactivity,40 neural plasticity,41 and/or remyelination42 as suggested by MR spectroscopic studies.
This study has limitations. The nonrandomized controlled design was due to the interventional limitations (eg, medical therapy alone suited patients with total ICA occlusion or those older than 70 years of age with tortuous vessels) and personal hesitation for intervention. Therefore, currently ongoing large-scale randomized controlled trials such as CREST-2 are warranted to determine long-term differences in efficacy between optimal medical therapy alone and combined revascularization therapy for stroke prevention (primary outcome) and cognitive preservation (secondary outcome) in patients with asymptomatic severe carotid stenosis. However, this small single-center trial provides new evidence of the benefit-risk balance for revascularization therapy and proposes a possible connectivity target for treating cognitive dysfunction in these patients. Furthermore, we did not assess the plaque-related microemboli and cerebrovascular reperfusion. Successful restoration of cerebral hypoperfusion was shown to correspond to the cognitive improvement after CAS.17 Additional transcranial emboli detection and perfusion imaging may help to elucidate the therapeutic mechanisms underlying cognitive and/or connectivity changes. Last, we cannot exclude the short-term placebo effects of subjective dizziness alleviation in the stent-placement group or a superimposed vestibular component in these patients.
Conclusions
Patients with severe asymptomatic carotid stenosis showed subjective dizziness alleviation in association with greater increases in microstructural connectivity at the posterior corpus callosum and periventricular white matter by aggressive medical therapy plus successful revascularization compared with aggressive medical therapy alone. However, the cognitive benefit was insignificant between groups at 3 months after treatment in our study. Unlike neurodegenerative causes of cognitive impairment, vascular damage is preventable and treatable. Our results suggest the feasibility of combined medical and revascularization treatment in severe asymptomatic carotid stenosis for limiting cognitive decline, possibly through ancillary connectivity enhancement. Large long-term controlled studies are warranted to provide a risk-benefit assessment for prophylactic carotid revascularization.
Acknowledgments
We gratefully acknowledge Wen-Yung Sheng for providing statistical advice. We also thank the Clinical Research Core Laboratory of Taipei Veterans General Hospital for providing experimental space and facilities.
Footnotes
Disclosures: Chun-Jen Lin—RELATED: Grant: Taipei Veterans General Hospital, Taiwan (V103C-171, V104C-059, V105B-029).* I-Hui Lee—RELATED: Grant: Ministry of Science and Technology, Taiwan (MOST103–2320-B-075–002, MOST103–2314-B-075–008, MOST104–2320-B-075–001), Taipei Veterans General Hospital, Taiwan (V103C-171, V104C-059, V105B-029)*; Support for Travel to Meetings for the Study or Other Purposes: Ministry of Science and Technology, Taiwan.* *Money paid to the institution.
This work was sponsored by Taiwan Ministry of Science and Technology (MOST 103-2320-B-075-002, MOST 103-2314-B-075-008, MOST 104-2320-B-075-001) and the Taipei Veterans General Hospital (V103C-171, V104C-059, V105B-029) in Taiwan.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received October 16, 2015.
- Accepted after revision March 10, 2016.
- © 2016 by American Journal of Neuroradiology