Abstract
INTRODUCTION: Functional MR imaging is increasingly being used for presurgical language assessment in the treatment of patients with brain tumors, epilepsy, vascular malformations, and other conditions. The inherent complexity of fMRI, which includes numerous processing steps and selective analyses, is compounded by institution-unique approaches to patient training, paradigm choice, and an eclectic array of postprocessing options from various vendors. Consequently, institutions perform fMRI in such markedly different manners that data sharing, comparison, and generalization of results are difficult. The American Society of Functional Neuroradiology proposes widespread adoption of common fMRI language paradigms as the first step in countering this lost opportunity to advance our knowledge and improve patient care.
LANGUAGE PARADIGM REVIEW PROCESS: A taskforce of American Society of Functional Neuroradiology members from multiple institutions used a broad literature review, member polls, and expert opinion to converge on 2 sets of standard language paradigms that strike a balance between ease of application and clinical usefulness.
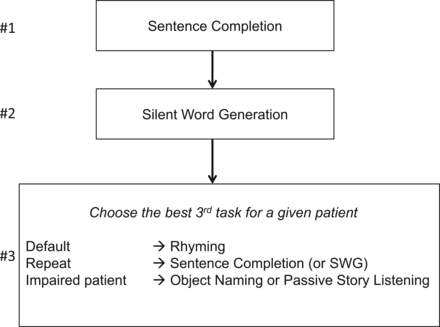
ASFNR RECOMMENDATIONS: The taskforce generated an adult language paradigm algorithm for presurgical language assessment including the following tasks: Sentence Completion, Silent Word Generation, Rhyming, Object Naming, and/or Passive Story Listening. The pediatric algorithm includes the following tasks: Sentence Completion, Rhyming, Antonym Generation, or Passive Story Listening.
DISCUSSION: Convergence of fMRI language paradigms across institutions offers the first step in providing a “Rosetta Stone” that provides a common reference point with which to compare and contrast the usefulness and reliability of fMRI data. From this common language task battery, future refinements and improvements are anticipated, particularly as objective measures of reliability become available. Some commonality of practice is a necessary first step to develop a foundation on which to improve the clinical utility of this field.
ABBREVIATIONS:
- AG
- Antonym Generation
- ASFNR
- American Society of Functional Neuroradiology
- BOLD
- blood oxygen level–dependent
- CVR
- cerebrovascular reactivity
- ECS
- electrocortical stimulation
- NVU
- neurovascular uncoupling
- ON
- Object Naming
- LI
- laterality index
- PSL
- Passive Story Listening
- SC
- Sentence Completion
- SWG
- Silent Word Generation
The use of fMRI in the presurgical assessment of language function, especially in patients with brain tumors, vascular malformations, or epilepsy, has become standard throughout numerous institutions in North America, Europe, and other parts of the world, and the reliance on this technology is increasing.1 fMRI offers a valuable noninvasive means of assessing language function lateralization and localization, which complements, or in some cases, obviates intraoperative electrocortical stimulation (ECS) mapping.2,3 The Organization of Human Brain Mapping Committee on Best Practice in Data Analysis and Sharing has recently published a white paper that addresses fMRI research reproducibility through transparency of trial design, tools used for data manipulation, and reporting.4
Beyond research reporting standards, the American Society of Functional Neuroradiology (ASFNR) perceives the need for increased standardization in clinical practice in an attempt to enhance the communicability of how we assess our patients and the meaningfulness of our imaging findings and reports. Functional MR imaging involves numerous processing steps, which vary among manufacturers of fMRI systems, and this complexity is compounded by institution-unique methods for patient training, paradigm choice, and postprocessing. Data sharing is necessary for research integrity and scientific transparency, but current practice variability obscures reproducibility and hinders adequate interinstitutional sharing of information. This widespread variability thereby limits collective progression, particularly in the presurgical assessment of language function—one of the most important applications of fMRI in clinical practice.
Since April 2013, the ASFNR has hosted a monthly teleconference in which clinical fMRI practitioners from across North America have presented presurgical mapping cases from their various practices for educational purposes. This recurring teleconference has highlighted the existing practice variability between institutions. However, it has also offered a regularly occurring, accessible line of communication that has provided impetus for converging practice parameters with a view toward enhanced validation of imaging methodologies, data sharing, and knowledge growth. Consequently, the ASFNR Clinical Practice Committee decided that to strengthen the value of preoperative fMRI language assessment, an ASFNR-approved set of standardized language paradigms should be developed.
Because language can be represented across phonologic, orthographic, semantic, pragmatic, and discourse dimensions and 1 task cannot simultaneously activate all of these aspects, multiple tasks are recommended to provide a more sensitive and specific map of language function that will aid in surgical planning.5,6 There are a number of desirable features for a standardized fMRI task battery: 1) The ideal fMRI language task paradigm set needs to be appropriately challenging for the patient to produce ample activation without being so difficult as to overwhelm a neurologically compromised or otherwise challenged individual; 2) the tasks need to provide an appropriate balance of sensitivity and specificity for language-related activation; and 3) there needs to be the ability to provide both reliable interhemispheric language lateralization as well as intrahemispheric localization of both expressive and receptive language sites, such as the Broca or Wernicke area, with respect to intracerebral lesions.
Language Paradigm Review Process
Members of an ASFNR Language Paradigm Taskforce were invited to join this project following discussions with the ASFNR Clinical Practice Committee and Executive Committee in an attempt to form a group of interested participants with expertise in the field, a historical perspective of how fMRI has evolved to its current state, and insight into how fMRI must continue to change to further the field.
As a first step, the members of the taskforce discussed their own institution's paradigm choices, practice parameters, and the value of combining results from multiple paradigms. From these discussions, project goals and scope were distilled, including the concept of deriving standardized recommendations that all institutions would hopefully adopt. Initially, the group decided to limit the first iteration of standard paradigms to visually presented paradigms for adult patients with a seventh-grade-equivalent or greater reading ability. Subsequently, it became clear that there was a clinical need to address the pediatric population and adults with limited reading ability, and recommendations for these populations were assessed and developed.
The entire ASFNR membership was polled as to how many language-assessment fMRIs per month were performed at their various institutions to determine the most commonly used language paradigms. The poll attempted to gauge the willingness of ASFNR members to adopt a common set of language paradigms. Poll results were assessed by the taskforce, and an attempt was made to balance current practice preferences across the nation with evidence-based data regarding the variable strengths and weaknesses of various language paradigms. The goal was to choose complementary paradigms that would be reasonably applicable to the greatest number of patients. By balancing current practice and scientific evidence, we hoped to motivate adoption of the standardized task battery by making the required change as simple as possible for the practicing members of the society while at the same time being guided by the scientific evidence. This goal necessitated a literature review for each of the most commonly used language paradigms.
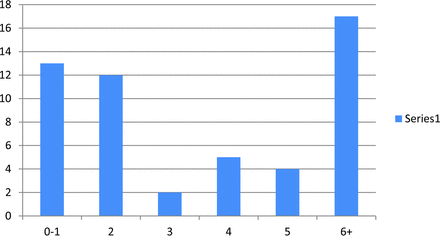
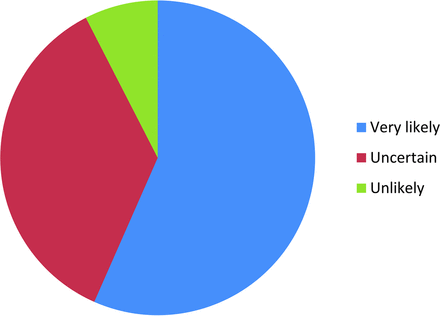
Two-hundred fifty-nine requests for surveys were sent out with 6 e-mail addresses failing. Fifty-three of 253 responded (21%). Figure 1 depicts the number of fMRIs performed per month for language assessment at different institutions. Fifty-seven percent (30/53) of the responders reported being very likely to adopt language paradigm algorithms, and only 8% (4/53) reported being unlikely to adopt ASFNR recommendations (Fig 2).
fMRIs performed per month for language assessment at different institutions (about one-third of responders do 6+ per month).
Fifty-seven percent (30/53) of responders reported being very likely to adopt language paradigm algorithms, and only 8% (4/53) reported being unlikely to adopt ASFNR recommendations.
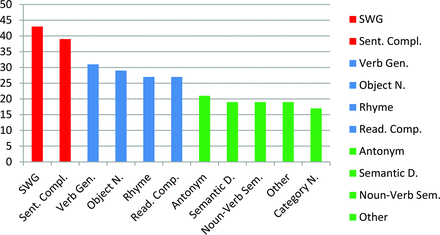
Results of the ASFNR poll produced 3 tiers of commonly used paradigms across the nation (Fig 3). In the poll, Silent Word Generation (SWG) and Sentence Completion (SC) stood out as the most frequently employed tasks while Verb Generation, Object Naming, Rhyming, and Reading Comprehension seemed to coalesce in a second-tier group. Because SWG and SC were the most commonly used paradigms and also had support in the literature for being reliable and useful, these tasks were favored to form the core of the standard language task battery. However, because a combination of language paradigms has been shown to increase sensitivity and specificity, a third task was considered desirable.7,8 Nevertheless, converging on a single choice for that third task proved to be more difficult because the taskforce wanted to maintain flexibility for the radiologist to tailor an examination to the patient's specific clinical scenario.9,10
The most commonly used language paradigms were sorted into 3 tiers with the total number of imagers by using the paradigms represented on the y-axis. The first tier included SWG and Sentence Completion. The second tier included Verb Generation, Object Naming, Rhyming, and Reading Comprehension. The third tier included Antonym Generation, Semantic Decision, Noun-Verb Semantic Association, Category Naming, and other.
Once the paradigm recommendations were decided upon, the taskforce then converged on the specific scanning parameters and stimuli for the paradigms such that the tasks would be vendor-neutral.
ASFNR Recommendations
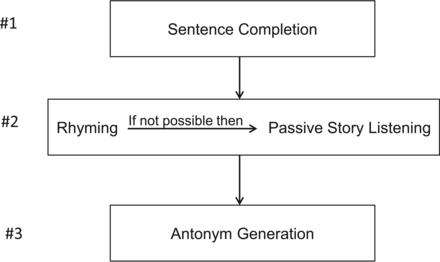
Following an analysis of the poll results as well as literature review regarding each of the paradigm tasks mentioned in the poll, the ASFNR taskforce developed language algorithms for both adults and pediatric subjects (Figs 4 and 5).
Adult algorithm for presurgical language fMRI.
Pediatric algorithm for presurgical language fMRI.
The default adult algorithm includes SC, SWG, and Rhyming. However, to satisfy the need to customize the analysis to the specific clinical scenario, the radiologist may choose to drop Rhyming and repeat the SC or SWG task as a means of confirming and correlating activations between time courses. SC most often offers more robust language area activation than SWG; thus, it would be the most appropriate task to repeat. In patients who may have difficulty adequately performing the SC or SWG tasks, the radiologist may choose either the Object Naming (ON) or Passive Story Listening (PSL) tasks. This also allows customization in that ON is primarily an expressive task and PSL is primarily receptive.
The default pediatric algorithm includes SC, Rhyming, and Antonym Generation (AG), but for those patients unable to adequately perform Rhyming, the PSL task should be used instead. In general, this pediatric algorithm should apply to most patients 5–11 years of age, but each patient's ability to adequately perform tasks should be assessed before the scan.
In addition to the language-specific tasks, breath hold fMRI may enhance the fMRI language task battery by identifying areas of potentially false-negative activation. By elevating blood carbon dioxide levels, a breath hold task measures cerebrovascular reactivity throughout the brain. It can therefore help demonstrate potential neurovascular uncoupling or confounding susceptibility artifacts as regions of absent or reduced blood oxygen level–dependent (BOLD) signal in response to breath holding, thereby clarifying regions of false-negative language activation. Due to atypical postprocessing requirements for this task, inclusion of the breath holding task is not considered to be a required component of the language task battery but is nonetheless recommended if available at an institution, to assess potential loss of sensitivity to the BOLD signal, which would influence the confidence level of the interpretation.
Discussion
We have generated an adult and a pediatric algorithm to be used as the standard paradigms for presurgical language fMRI assessment (Figs 4 and 5). The scanning parameters for each of these tasks are listed in the Appendix. The ASFNR contends that all institutions should be performing the same paradigms because disparity of practice limits progress in the field and converging clinical practice is necessary to ensure that we all provide the best possible care to our patients. The following sections provide a brief review of each of these tasks, and the Table summarizes individual task utilities.
Summary of individual task usefulness
Sentence Completion Task
The Sentence Completion language task is one of the many language paradigms that can be used for language localization and hemispheric lateralization for identifying the primary language cortex. SC is a semantic language paradigm that is effective in activating the superior temporal gyrus in the Wernicke area.11 SC can also activate the Broca area in the dominant hemisphere because the task requires both receptive and expressive language processing, though activation of Broca is slightly less robust than in Wernicke.11 Additionally, the pattern of activation with SC is less lateralized to Broca compared with SWG because of involvement of the homologous right hemisphere in speech-comprehension tasks, which invoke executive processing.7,12 Since verbal comprehension tasks such as SC involve visual processing, activation of the ventral language stream, including the visual word form area, can also be seen.13,14 SC has been found to produce increased activation in both temporal and frontal regions compared with a word generation task, suggesting more robust activation of language networks because it combines language comprehension as well as production in a naturalistic fashion.15
The control task for SC uses gibberish sentences devoid of the semantic, syntactic, and lexicalization demands that are present in the active task.
Silent Word Generation Task
Silent Word Generation is a commonly applied clinical language fMRI paradigm for presurgical mapping with extensive comparisons with Wada testing.11,14,16⇓⇓–19
Like most word generation paradigms, SWG tasks activate mainly frontal lobe language and cognitive support areas but are less consistent activators of temporal language regions.11,14,19,20 Specifically, SWG tasks yield reliable activation of the inferior frontal gyrus, dorsolateral prefrontal cortex, and superior frontal gyrus, with variable activation in the anterior cingulate gyrus, presupplementary motor area, and inferior, middle, and superior temporal gyri.14,16,17,21,22
Several studies have validated the SWG paradigm for both language lateralization and localization.21⇓⇓⇓⇓–26 For example, Yetkin et al23 reported 100% sensitivity of fMRI by using a SWG task for activation within 20 mm of the electrically stimulated cortical site during ECS and 86% sensitivity within 10 mm in a mixed series of 28 patients with predominantly epilepsy (n = 22) and cerebral lesions. Brannen et al24 studied the reliability, precision, and accuracy of word generation tasks in mapping the Broca area in 34 patients with cerebral lesions by comparing regions of activation with awake (ECS) mapping of speech function during craniotomy. They noted that SWG tasks activated Brodmann areas 44 or 46 either individually or both unilaterally or bilaterally in most patients in the series with variable activation of Brodmann areas 9 and 45. Activation was noted in the same gyri when the patient performed a second iteration of the SWG task, and speech areas located with ECS coincided with areas of the brain activated with the SWG task. Comparing hemispheric language dominance by using an SWG task with results of Wada testing in a large series of 100 patients with epilepsy, Woerman et al27 found 91% concordance between both tests. Similarly, Sabbah et al21 demonstrated that fMRI language lateralization based on the SWG was concordant with the Wada test in 19 of 20 patients with intractable partial epilepsy.
Pillai's group compared the localization (as locally detectable statistically significant percentage signal change) and lateralization among 5 language paradigms: SWG, Sentence Completion, Visual Antonym Pair, Auditory Antonym Pair, and Noun-Verb Association in 5 ROIs: inferior frontal gyrus, middle frontal gyrus, and superior frontal gyrus for expressive language activation; and middle temporal gyrus and superior temporal gyrus for receptive language activation in a group of 12 healthy volunteers.14 The results of this study found that SWG was the most robust paradigm for language localization and the most effective for determining language lateralization in the expressive ROIs of the dominant language hemisphere. In contrast, the analysis of patterns of activation in the receptive ROIs in the temporal gyri demonstrated a weaker BOLD percentage signal change for SWG tasks in both hemispheres when compared with the other 4 paradigms that include some form of language comprehension in the active blocks of the paradigm, which are localized in the angular and middle temporal gyri.28
A typical control used for the SWG task employs nonsense symbols without the phonemic fluency-processing demands of the active task.
Rhyming Task
Rhyming is considered a phonologic task that has been shown to robustly activate the Broca area in the dominant hemisphere.17 Rhyming also activates the Wernicke area though not as strongly as seen in Broca.11 In addition to the above regions, Rhyming has been shown to activate the inferior parietal lobule, the dorsolateral prefrontal cortex, and posterior lateral gyrus.29 Rhyming and SWG are the 2 paradigms found to be most helpful for language lateralization compared with Sentence Completion and Noun-Verb Association.11 As with Sentence Completion, there is activation of the posterior temporo-occipital regions due to involvement of the visual processing pathways.13
Studies comparing the relative effectiveness of different commonly used expressive and receptive language paradigms by using both threshold-independent and threshold-dependent methods in patients with brain tumors demonstrated that SWG and Rhyming were more helpful for language lateralization than SC or Noun-Verb Association.11,19 The Rhyming task has several advantages over the SWG task: It results in more specific activation of language areas than SWG, has a higher mean laterality index (LI) value, and is less threshold-dependent than SWG for determining the LI.11,17,19 Nevertheless, both SWG and Rhyming demonstrate adequate language lateralization, even in a subgroup of patients with brain tumors located in the left hemisphere and in the frontal or parietal lobes.19 This has clinical implications since the presence of susceptibility artifacts or neurovascular uncoupling associated with tumors in the dominant hemisphere could affect the strength of the BOLD signal.
The control task most often employs nonsense symbols wherein the patient is asked whether 2 sets of symbols are oriented in the same fashion, which allows monitoring of the patient's attention and task engagement without stimulating phonologic processing like the active task.
Object Naming Task
Simple Object Naming is an expressive language paradigm in which the subject is shown an object and is asked to silently name the presented object.30⇓–32 The control block consists of a nonsense symbol to exclude the visual component from the activation maps. The objects are presented in black and white.
ON tasks yield activation in the inferior frontal gyrus, middle frontal gyrus, and ventral occipitotemporal cortex, with variable activation in the posterior temporoparietal cortex.30,31,33,34 Several studies have validated the ON paradigm for both language lateralization and localization.8,19,31,34⇓–36 For example, Rutten et al8 reported that a combination of 3 different fMRI language tasks (ON, Verb Generation, and Sentence Processing) were able to localize critical language areas with 100% sensitivity and 61% specificity when compared with ECS mapping in a group of patients with epilepsy. Similarly, Hirsch et al31 demonstrated 100% concordance of localization between fMRI and ECS when using ON and word-listening tasks in neurosurgical candidates, most of whom had brain tumors,36 and Pouratian et al35 demonstrated 100% sensitivity and 67% specificity for frontal language regions when correlating ECS mapping and fMRI in patients with vascular malformations who performed Object Naming, Word Generation, and auditory-response naming tasks.
Although ON provides relatively good localization predominantly of frontal language regions, the general consensus is that the activation pattern associated with ON does not allow as effective hemispheric language lateralization as with other expressive language paradigms such as SWG, Verb Generation, and Rhyming.19,34 Moreover, like other covert paradigms, the ON task does not allow monitoring of patient responses. Despite these limitations, the ON task is easy for most patients to perform and is therefore often utilized in patients with cognitive impairment or pediatric patients, and it is frequently used during intraoperative cortical stimulation.
Antonym Generation Task
This word generation paradigm typically presents the patient with 1 word at a time, and the patient is asked to silently think of the word that means the opposite. Typically, during each activity period, 5–10 words are sequentially presented on the screen, and during the rest period, a black blank screen is presented with a white crosshair in the center for visual fixation.
This paradigm stimulates phonologic, working memory, lexical search, semantic, and orthographic processes of the speech and language system. Functional maps typically demonstrate activation in the inferior frontal gyrus, middle frontal gyrus, premotor cortex, and frequently posterior peri-Sylvian speech areas (posterior aspect of the superior temporal lobe, supramarginal and angular gyri). Frequent weak activation is also seen in the posterior aspect of the inferior temporal lobe, possibly due to engagement of the visual word form systems.37⇓⇓–40 Because there is no control for visual sensory processing during the resting phase, frequently, bilateral primary visual cortex activation is also seen.
The Antonym Generation task is reported to provide a higher percentage BOLD signal change in the Broca area (versus baseline) compared with the Antonym Decision task (versus baseline).41 It is reported that activation of the speech areas and speech lateralization in the various Word Generation tasks (letter, category, antonym word generation) is comparable.42 However, in our experience with pediatric patients, the Antonym Generation paradigm does much better in terms of the extent of activation in the speech areas and patient compliance versus letter Word Generation tasks; thus, SWG was not included in the pediatric algorithm. One of the limitations of this paradigm is that given the design, it is difficult to assess patient performance.
Passive Story Listening Task
Passively listening to an aurally presented story involves multiple aspects of language function, including syntactic processing and word recognition. This task offers the advantage of being relatively easy to accomplish for young children or adults whose functional impairments limit their ability to adequately partake in more complex language paradigms.
Passive Story Listening has been shown in pediatric populations to produce activation in the bilateral superior temporal gyrus, bilateral superior frontal gyrus, and left posterior superior temporal gyrus.43⇓⇓–46 The ease of performing PSL in young or impaired adult patients must be balanced with the lack of a task performance metric and its relatively weak language activation. There is no way to know if the patient is actually listening and attending to the story during the scan, but patients may be asked to recount the stories they heard following the scan as an indirect means of assessing compliance.
Scanner noise can impede the quality and magnitude of language activation during aurally presented tasks.47 Vannest et al48 compared similar PSL and active-response tasks and found that both produced language activation in similar patterns of the frontal and temporal lobes but the active-response task produced more dorsolateral prefrontal activation, likely due to engagement of working memory and attention during the comprehension questions. No significant difference in hemispheric lateralization was observed. Although adding an active-response component increased memory and attention engagement as well as effect size in the left inferior frontal gyrus, we chose the simpler version of this task because it still provides adequate activation and lateralization of language function while applying to the greatest range of children and/or impaired adult patients. The control task typically involves reversed playback of the same story to remove semantic processing from the sounds presented or simply playing tones.
Breath Hold Task
One task that may be performed for quality control purposes as part of standard clinical presurgical mapping fMRI examinations is the breath hold task. Using such a task, one can alternate brief breath hold periods with periods of normal self-paced respiration in a standard block design paradigm. A general linear model analysis can be performed to evaluate percentage BOLD signal change occurring during the hypercapnia state relative to the normocapnia condition. Although one may utilize either end-inspiratory or end-expiratory breath holds to induce the transient hypercapnia, and both approaches have relative strengths and weaknesses (eg, some studies have shown greater reproducibility with the latter approach), we have found that in general, the end-inspiratory technique is easier for patients to perform and can be performed even by neurologically debilitated patients.49⇓–51 The purpose of this task is to evaluate cerebrovascular reactivity (CVR) in a relatively quick, easy, and effective manner without the need for exogenous controlled gas administration as is frequently used for quantitative CVR research studies.52⇓⇓–55 Although emerging resting-state BOLD imaging approaches have been suggested as well for assessment of CVR, there remains some controversy regarding the reliability of such methods such as resting-state fluctuation of amplitude, and thus they cannot be recommended currently.56⇓–58
The main value of this method is its utility in detecting neurovascular uncoupling potential in patients with focal resectable brain lesions such as tumors, vascular malformations, or cortical developmental abnormalities. Neurovascular uncoupling (NVU) refers to the breakdown of the normal neurovascular coupling cascade related to factors such as tumor angiogenesis, astrocytic dysfunction, neurotransmitter, or other biochemical dysfunction or abnormalities of intralesional or perilesional hemodynamics. Generally, the final step in the cascade involves the vascular response related to regional neuronal activation, and thus most cases of NVU manifest as abnormally decreased or absent regional vascular reactivity within or immediately adjacent to the brain lesion of interest.49,59⇓⇓⇓–63 Determining potential NVU increases the clinical utility of fMRI by uncovering false-negative language activations in the eloquent cortex.
Conclusions
The ASFNR proposes that institutions performing presurgical language fMRI consider adopting these recommended paradigm algorithms as standard clinical practice. The algorithms were created with an attempt to balance paradigms that primarily activate frontal/expressive regions (SWG, AG, ON) and those that primarily activate temporal/receptive areas (SC, PSL). Paradigms were also chosen to balance varying levels of sensitivity and specificity as well as strengths in lateralization and localization. The algorithms are meant to provide easily adopted, clinically useful paradigms that would apply to the greatest number of clinical scenarios. The tasks can be downloaded for free at https://www.asfnr.org/paradigms/.
Using common paradigms is only the first necessary step in practice convergence in an effort to reduce the widespread variability in clinical fMRI. By design, it is expected that these recommendations will change with time. The commonality that they provide will allow interinstitutional comparisons that will eventually provide insights into the best methodologies, enhance the value of every institution's fMRI program, and provide a common frame of reference from which the field can advance.
Radiologists, especially those who have innovated and created fMRI tasks that are used on a daily basis in their respective practices, may be hesitant to discard their favorite, personally developed language tasks in the name of standardization and convergence. Moreover, interinstitutional standardization runs against the normal business model of attempting to differentiate one's practice from one's competitors. However, adopting standard paradigms is the natural evolution of a technology that is initially replete with locally derived, often ingenious methods that because of their uniqueness, unfortunately limit meaningful communication with other professionals as well as the ability to generalize clinical and research contributions beyond one's own walls.
Converging language paradigms are only a small component of fMRI practice. Ongoing variability in terms of MR imaging scanner models, patient training sessions, and postprocessing techniques will continue to impact the generalizability of fMRI results. Institutions will still be able to differentiate themselves through interpretation quality and other patient care metrics. While there are varying degrees of support for these language paradigms in the literature, the most compelling aspect in favor of adopting this set of tasks is that they are already widely used in clinical practice.
Appendix: Minimum Acceptable Scanner Parameters for Performing ASFNR-Recommended Language Paradigms
The actual content of each paradigm will be available for download on the ASFNR Web site (https://www.asfnr.org/paradigms/).
Task list
Sentence Completion
Silent Word Generation
Rhyming
Antonym Generation
Passive Story Listening
Object Naming
1) Sentence Completion Task Parameters*
Gradient-echo EPI sequence
Field strength: 3T
TR: 2000 ms
TE: 30 ms
Matrix: 64 × 64
FOV: 24 cm
Section thickness: 4 mm
Parallel factor: 2
Scan length: 4 minutes
Font: 42-point Times New Roman font (on a standard PowerPoint slide) in white with a black background.
Stimuli: The patient is shown incomplete sentences and is instructed to subvocally think of a word or words to complete the sentence. If there is time, the patient should continue to think of alternate words that complete the sentence before the next incomplete sentence being presented. Four sentences per 20 seconds. This stimulus timeframe is repeated 6 times.
Control: Patient views nonsense sentences. Four nonsense sentences per 20 seconds.
*From Faro et al multicenter ongoing trial.
2) Silent Word Generation Task Parameters*
Gradient-echo EPI sequence
Field strength: 3T
TR: 2000 ms
TE: 30 ms
Matrix: 64 × 64
FOV: 24 cm
Section thickness: 4 mm
Parallel factor: 2
Scan length: 4 minutes
Font: Times New Roman font size 117 (on a standard PowerPoint slide), white font on black background.
Stimuli: A test of phonemic fluency wherein a patient is shown a single letter and asked to subvocally think of as many words that start with that letter as they possibly can before the image changes to either the control or the next letter. Ten seconds per letter. The patient can be instructed to press a button with each word they think of as a means of monitoring their active engagement with the task.
Control: Patient views nonsense symbols (10 seconds per nonsense symbol).
Six cycles = 4 minutes
*From Faro et al multicenter ongoing trial.
3) Rhyming Task Parameters
Gradient-echo EPI sequence
Field strength: 3T
TR: 2000 ms
TE: 30 ms
Matrix: 64 × 64
FOV: 24 cm
Section thickness: 4 mm
Parallel factor: 2
Scan length: 4 minutes
Font: Times New Roman font size 200 (on a standard PowerPoint slide), white font on black background.
Stimuli: The patient is shown 2 words, one on top of the other, and asked to respond with a button press if the 2 words rhyme. If the words do not rhyme, no button press should occur. Five word pairs per 20 seconds.
Control: Two sets of 5 differently oriented lines (one set on top of the other) are shown to the patient, who is asked to respond with a button press if the 2 sets are oriented in an identical fashion. If not, no button press is needed.
4) Antonym Generation Task Parameters
Gradient-echo EPI sequence
Field strength: 3T
TR: 2000 ms
TE: 30 ms
Matrix: 64 × 64
FOV: 24 cm
Section thickness: 4 mm
Parallel factor: 2
Scan length: 2 minutes 40 seconds
Font: Times New Roman font size 44 (on a standard PowerPoint slide), white font on black background.
Stimuli: The patient is shown single words and asked to generate an opposite-meaning word in his or her mind. Ten words per block and a total of 4 blocks. Each word remains on the screen for 2 seconds.
Control: Simple crosshair in the center of the screen lasts for 20 seconds for each block.
5) Passive Story Listening Task Parameters
Gradient-echo EPI sequence
Field strength: 3T
TR: 2000 ms
TE: 30 ms
Matrix: 64 × 64
FOV: 24 cm
Section thickness: 4 mm
Parallel factor: 2
Scan length: 4 minutes
Font: This is an aurally presented paradigm.
Stimuli: The patient listens to 20 seconds of a story (selected passages from “The Tale of Peter Rabbit,” by Beatrix Potter). Alternates with control for a total of 4 minutes.
Control: The patient listens to 20 seconds of the same story played backwards.
(Reading performed by Ann Marie Rydberg)
6) Object Naming Task Parameters
Gradient-echo EPI sequence
Field strength: 3T
TR: 2000 ms
TE: 30 ms
Matrix: 64 × 64
FOV: 24 cm
Section thickness: 4 mm
Parallel factor: 2
Scan length: 4 minutes
Font: Similarly sized images and symbols will be shown (no text).
Stimuli: White outline images of objects on a black background will be shown to the patient who will be asked to covertly/silently name the object. One object is presented approximately every 3 seconds.
Control: Nonsense symbols are shown to the patient, and he or she does nothing other than attend to what is being shown. One symbol is presented approximately every 3 seconds.
Breath Hold Task Parameters
(Recommended but not part of the specified algorithms)
Gradient-echo EPI sequence
Field strength: 3T
TR: 2000 ms
TE: 30 ms
Matrix: 64 × 64
FOV: 24 cm
Section thickness: 4 mm
Parallel factor: 2
Scan length: 4 minutes 20 seconds
Font: Times New Roman font size 44 (on a standard PowerPoint slide), white font on black background.
Technique: Slow controlled 4-second inspiration followed by a 16-second breath hold. This is then followed by a 40-second block of self-paced normal breathing. This cycle is repeated 4 times with an additional 20-second period of normal breathing for a total task duration of 4 minutes 20 seconds.
From: Pillai JJ, Mikulis DJ. Cerebrovascular reactivity mapping: an evolving standard for clinical functional imaging. AJNR Am J Neuroradiol 2015;36:7-13.
Footnotes
J.J. Pillai and K. Welker are co-senior authors.
Indicates open access to non-subscribers at www.ajnr.org
References
- © 2017 by American Journal of Neuroradiology