Abstract
BACKGROUND AND PURPOSE: Flow diversion is an established method to treat complex intracranial aneurysms. The natural history of flow-diversion treatment failure resulting in aneurysm remnants is not well-defined. We aimed to delineate the clinical and angiographic features of this entity.
MATERIALS AND METHODS: Review of a prospectively maintained Pipeline Embolization Device data base from inception to October 2017 was performed for aneurysms that demonstrated residual filling on follow-up imaging. Procedural and follow-up clinical details were recorded. Independent, blinded, angiographic assessment of occlusion was performed on the basis of the O'Kelly-Marotta scale. Aggregated outcomes were analyzed using the Fisher exact and Mann-Whitney U tests for categoric and continuous variables, respectively (statistical significance, α = .05).
RESULTS: During the study period, 283 sequential patients were treated; 87% (246/283) were women. The median patient age was 55 years (interquartile range, 47–65 years). Six-month follow-up imaging was available in 83.7% (237/283) of patients, which showed 62.4% (148/237) complete occlusion (class D, O'Kelly-Marotta grading scale). Adjunctive coiling (P = .06), on-label Pipeline Embolization Device use (P = .04), and multiple device constructs (P = .02) had higher rates of complete occlusion at 6 months. Aneurysm remnants were identified in 25 cases on long-term follow-up imaging (median, 16 months; interquartile range, 12–24 months). No patient with an aneurysm remnant after flow diversion presented with delayed rupture or other clinical sequelae, with a median clinical follow-up of 31 months (interquartile range, 23–33 months).
CONCLUSIONS: Aneurysm remnants after flow diversion are infrequent with minimal clinical impact. When appropriate, the presence of overlapping devices and possibly adjunctive coiling may result in higher rates of complete occlusion.
ABBREVIATIONS:
- IQR
- interquartile range
- OKM
- O'Kelly-Marotta grading scale
Flow diversion with the Pipeline Embolization Device (PED; Covidien, Irvine, California) was first reported in 2008.1 Since that time, multiple trials2,3 and retrospective case series4,5 have supported the role of the PED in the treatment of complex intracranial aneurysms. Current follow-up data show high rates of angiographic occlusion at 5 years with only a 4.8% rate of aneurysm persistence and no evidence of recanalization of previously occluded aneurysms.6 In contrast, coil embolization of intracranial aneurysms results in higher rates of aneurysm persistence.7 Increasingly, natural history data describing the angiographic8 and clinical outcomes9 of aneurysm remnants after endovascular therapy are now available. Preliminary evidence suggests that the type of aneurysm remnant (neck versus body filling) dictates the rate of recanalization after coil embolization (with neck filling having lower rates of recanalization compared with body filling)8 and remnants of previously ruptured aneurysms are at a higher risk of rerupture.9 However, long-term imaging and clinical data with respect to aneurysm remnants after flow diversion remain sparse. Our goal was to evaluate the longitudinal angiographic and clinical outcomes of aneurysm remnants after flow diversion with the PED.
Materials and Methods
A prospectively maintained institutional (Emory University) data base of patients treated with the PED from 2011 through October 2017 was searched for patients who demonstrated residual filling on follow-up imaging. Patient-level information regarding aneurysm characteristics, procedural details, and clinical follow-up was collected into an electronic data base. The main exclusion criterion was ruptured aneurysms treated in the acute or subacute period (0–14 days). Collected data points included patient characteristics of age, sex, family history of aneurysms, tobacco use; aneurysm characteristics, including type, size, and location; and procedural details such as the number of devices used, adjunctive coiling, and clinical outcome along with the duration of both clinical and radiographic follow-up. If data were available at 6 months, changes to dual antiplatelet therapy after aneurysm occlusion status were noted. Incomplete occlusion status was defined as classes A–C based on the O'Kelly-Marotta (OKM) grading scale10 for assessment of aneurysms treated by flow diversion. Independent, retrospective review of follow-up imaging was completed by a fellowship-trained neuroradiologist (3 years of dedicated cerebrovascular experience) who did not participate in the initial procedure. Complete occlusion was defined as class D based on the OKM grading scale.
The association between demographic and clinical risk factors with incomplete occlusion and long-term clinical outcome for patients with incomplete occlusion versus patients with complete occlusion was evaluated using the Fisher exact and Mann-Whitney U test for categoric and continuous variables, respectively. The threshold of statistical significance was α = .05. All statistical analyses were performed using SPSS, Version 22 (IBM, Armonk, New York) and Excel 2007 (Microsoft, Redmond, Washington).
Results
During the study period, 296 interventions were performed in 283 patients to treat 294 aneurysms. Overwhelmingly, the patients treated were women (87%). The median patient age was 55 years (interquartile range [IQR], 47–65 years). Minimum 6-month follow-up imaging was available in 83.7% (237/283) of patients, which included either DSA (50.2%; 119/237), MRA and DSA (36.3%; 86/237), or MRA (13.5%; 32/237). Table 1 provides summary demographic data.
Basic clinical and imaging demographics
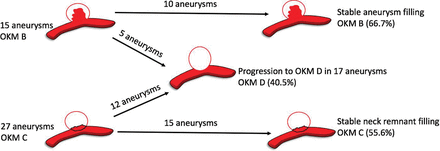
At 6 months, 62.4% (148/237) of aneurysms demonstrated complete occlusion (class D, OKM scale), 24.1% (57/237) of aneurysms had an entry remnant (class C, OKM scale), and 13.5% (32/237) of aneurysms had subtotal filling (class B, OKM scale). In patients with available late surveillance imaging at a median duration of 16 months (IQR, 12–24 months), progressive complete occlusion was observed in 40.5% (17/42) of aneurysms (class D, OKM scale). The most common dual antiplatelet regimen (73.4%; 174/237) at midterm follow-up imaging was 75 mg of clopidogrel and 325 mg of aspirin. Tapering of antiplatelet therapy was initiated by the treating physician only if progressive aneurysm occlusion was noted on midterm imaging and in the absence of in-stent intimal hyperplasia. The precise regimen was both patient- and operator-specific. Higher rates of progressive complete occlusion were observed in aneurysms with remnant necks (44.4%, 12/27; class C, OKM scale) as opposed to aneurysms with subtotal filling (33.3%, 5/15; class B, OKM scale), though this trend was not statistically significant (P = .53). Figure 1 demonstrates progressive occlusion rates of aneurysms with residual filling on late surveillance imaging in a pictorial format.
Occlusion status on late-surveillance follow-up imaging.
Procedural characteristics and aneurysm morphology played an important role in aneurysm occlusion on midterm (6 month) imaging follow-up. Adjunctive coiling during the initial PED placement resulted in higher rates of complete occlusion at 6 months (73.9%, 34/46 versus 58.6%, 99/169); this result almost reached statistical significance (P = .06). On-label use of the PED was associated with a higher rate of occlusion (73.0%, 54/74) than off-label use (58.5%, 93/159) (P = .04). In addition, deployment of >1 device resulted in higher rates of complete occlusion at 6 months (79.5%, 31/39) versus a single device (58.9%, 116/197) (P = .02). No correlation between wide-neck aneurysms (>4 mm; P = .29) or diameter (>10 mm; P = .52) and the rate of occlusion was found.
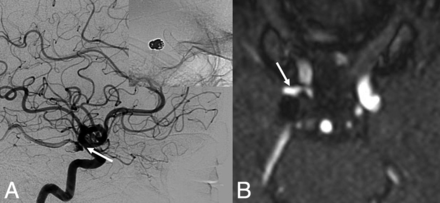
A total of 25 aneurysm remnants were available for clinical analysis based on last known follow-up imaging. No patient with an aneurysm remnant after flow diversion presented with delayed rupture or other clinical sequelae (median clinical follow-up, 31 months; IQR, 23–33 months). Details related to aneurysm remnants are noted in Table 2. In addition, angiographic follow-up demonstrated no progression of neck remnants. Figure 2 is one such representative case.
Characteristics of aneurysm remnants after flow diversion
Aneurysm persistence after flow diversion. A 67-year-old woman who had a previously ruptured right posterior communicating artery aneurysm with evidence of recanalization on the 6-month follow-up angiogram. She was treated with flow diversion for the neck recurrence. A 6-month follow-up DSA (lateral x-ray) after flow diversion demonstrates class C OKM grade (continued filling at the neck of the aneurysm) (white arrow). At last clinical and angiographic follow-ups at 3 years, there is no evidence of aneurysm rerupture or progression of the neck remnant on MRA (white arrow).
Discussion
Flow diversion has emerged as a paradigm shift in the treatment of intracranial aneurysms. As more data define characteristics associated with aneurysm persistence after flow diversion,11⇓–13 improved patient selection and procedural technique may increase long-term occlusion rates. Parent vessel remodeling resulting in aneurysm occlusion after flow diversion is a remarkably different mechanism compared with coil embolized aneurysms. In addition, high rates of aneurysm occlusion and durability of treatment6 make studying aneurysm remnants after flow diversion difficult. Therefore, the natural history of this entity is ill-defined. Our study emphasizes the importance of adjunctive coiling and overlapping devices when appropriate to achieve higher rates of occlusion on midterm imaging follow-up. Moreover, we find that the clinical impact of aneurysm remnants after flow diversion is benign, with no evidence of rerupture in our cohort.
Predictors of Occlusion after Flow Diversion
Adjunctive coil embolization during flow diversion has been previously described by many reports.14,15 The placement of loosely packed coils inside the aneurysm at the time of flow diversion provides an additional element of flow disruption to aid aneurysm thrombosis. This observation is confirmed in our study and falls in line with the literature: The presence of adjunctive coiling leads to greater rates of aneurysm occlusion on midterm follow-up imaging. Although our result is slightly underpowered to reach statistical significance (P = .06), Lin et al14 noted a higher proportion of complete occlusion with adjunctive coiling at the time of flow diversion in 29 patients compared with flow diversion alone in 75 patients (93.1% versus 74.7%; P = .03). High rates of occlusion on follow-up imaging were similarly replicated by Nossek et al15 because all aneurysms that underwent flow diversion and adjunctive coiling were occluded. Enthusiasm for adjunctive coiling has been tempered by a few studies16 reporting that robust coil packing during flow diversion can cause device occlusion due to mass effect and increased thrombogenicity from the large coil mass. However, we did not experience device occlusion or an increased rate of ischemic complications with adjunctive coiling. This outcome has been confirmed by multiple case series that show the equivalent risk profile of flow diversion with or without adjunctive coiling.14,15,17
Aneurysm occlusion after flow diversion is dependent on metal surface coverage.18 Constructs with multiple overlapping flow diverters placed across the aneurysm neck favorably increase surface coverage both in ex vivo19 and computational fluid dynamics models.20 Multiple studies corroborate these basic science observations, with both the Pipeline embolization device for the Intracranial Treatment of Aneurysms trial (PITA)3 and Pipeline for Uncoilable or Failed Aneurysms (PUFS)2 trials supporting the efficacy of using overlapping flow diverters to treat aneurysms. Similarly, we show that the use of multiple devices resulted in higher rates of occlusion compared with 1 device (P = .02). Understandably, increased metal surface coverage and number of devices may increase thrombogenicity and, as a result, thromboembolic complications.21 Chalouhi et al22 proposed a single-device rationale for treatment of aneurysms on the basis of decreased complication rates and equivalent rates of occlusion between single- and multiple-device constructs. On the contrary, a larger study by Brinjikji et al23 showed that in 906 treated aneurysms, only fusiform aneurysm morphology was independently associated with ischemic complications after multivariate analysis (P < .001). Given the conflicting data, larger trials will be required to determine the safety profile of overlapping devices.
Aneurysm Remnants after Flow Diversion
The natural history of remnants after coil embolization of a ruptured aneurysm is well-defined: The more complete occlusion on postprocedural DSA, the lower is the risk of rerupture.24 Increasingly, studies show that unruptured neck remnants after endovascular treatment (stent-assisted coiling/coiling) are both angiographically8 and clinically9 benign on long-term follow-up. Mascitelli et al8 showed that in 99 completely occluded aneurysms and 110 neck remnants after coil embolization, angiographic outcome was similar with very few recanalizations. Munich et al9 analyzed the rerupture rate in 626 aneurysms with residual filling on immediate posttreatment angiography. Ruptured aneurysms with neck remnants pose a high risk of rerupture (3.4%), while unruptured aneurysms with residual necks confer a very low risk of rupture (0.6%). Although these results may not directly correlate with aneurysm remnants after flow diversion, the benign nature of unruptured flow-diverted aneurysm neck remnants should be considered. In addition, the progressive occlusion, parent vessel remodeling, and stasis of flow within the aneurysm sac after flow diversion may be protective against progression of aneurysm neck remnants to definite recurrence. In 25 aneurysm remnants with a median clinical follow-up of 31 months, we did not observe any evidence of rerupture or neck progression. Moreover, in a recent report by Kan et al,25 none of 16 cerebral aneurysms that failed to occlude after flow diversion ruptured during the follow-up period (mean follow-up duration, 24 months). Like the results of Mascitelli et al and Munich et al, the results of Kan et al suggest that remnants of unruptured aneurysms, despite being treated by different devices, are benign.
Although these results are preliminary, the clinical implications of our findings can be considered. Treatment failures after flow diversion resulting in neck remnants or persistent aneurysm filling did not progress in our series. Therefore, these cases could be monitored with noninvasive temporal MRA imaging.26 If progression of an aneurysm is confirmed on DSA, then a treatment decision about a second device can be considered. In addition, antiplatelet therapy can be de-escalated in cases in which remnants have persisted past 12 months because endothelization of the stent construct has likely already occurred.
Limitations
The retrospective nature of this study and analysis from a single academic center introduces sampling bias and possibly limits external validity. In addition, other angiographic findings that may be associated with aneurysm persistence that were not measured include inflow angle to aneurysm ostium, vessel size arising from aneurysm neck, and degree of malapposition as measured by VasoCT (Philips Healthcare, Best, the Netherlands).27 Angiographic follow-up at the 6-month time point was relatively consistent for the entire cohort; however, long-term imaging follow-up (MRA/DSA) was variable and at the discretion of the treating neurointerventionalist. The variable regiment of long-term imaging follow-up of the treating physician introduces heterogeneity with respect to our results of occlusion rates on long-term follow-up. In addition, long-term imaging follow-up was not available for all patients (52.8%; 47/89) with aneurysm persistence at 6 months. Some patients were lost to follow-up (72.3%; 34/47), or no follow-up was available for miniscule aneurysm remnants (27.7%; 13/47) that were initially interpreted as complete thrombosis or a tiny remnant that would eventually thrombose.
Conclusions
Preliminary results suggest that aneurysm remnants after flow diversion are infrequent with minimal clinical impact. When appropriate, the presence of overlapping devices and possibly adjunctive coiling may result in higher rates of complete occlusion. Larger studies with long-term clinical follow-up will be needed to confirm these findings.
Acknowledgments
We thank Duk Soo Han, MPH, for statistical review.
Footnotes
Drs Thomas P. Madaelil and Jonathan A. Grossberg are co-first authors.
Disclosures: Jonathan A. Grossberg—UNRELATED: Consultancy: Cognition Medical, Comments: no fees, only stock options; Grants/Grants Pending: Georgia Research Alliance; Stock/Stock Options: equity in Neurotechnology Investors (NTI). Jacques Dion—UNRELATED: Employment: MicroVention/Terumo, Comments: employee, Vice President of Scientific Affairs; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: MicroVention/Terumo, Comments: as part of my employment duties. Raul G. Nogueira—OTHER RELATIONSHIPS: Stryker Neurovascular (DAWN Trial Principal Investigator, no compensation; TREVO Registry Steering Committee, no compensation; TREVO 2 trial Principal Investigator, modest; consultant, modest), Medtronic (SWIFT Trial Steering Committee, modest; SWIFT PRIME Trial Steering Committee, no compensation; STAR Trial Angiographic Core Lab, modest compensation), Penumbra (3D Separator Trial Executive Committee, no compensation), Cerenovus/Neuravi (ENDOLOW Trial Principal Investigator; EXCELLENT Registry Principal Investigator; ARISE 2 trial Steering Committee, no compensation; Physician Advisory Board, modest compensation), phenox (Physician Advisory Board, modest compensation), Anaconda (Physician Advisory Board, modest compensation), Genentech (Physician Advisory Board, modest compensation), Biogen (Physician Advisory Board, modest compensation), Prolong Pharmaceuticals (Physician Advisory Board, modest compensation), Allm Inc (Physician Advisory Board, no compensation), iSchemaView (speaker, modest compensation), Brainomix (research software use, no compensation), Sensome (research device use, no compensation), Viz.ai (Physician Advisory Board, stock options), Philips Healthcare (research software use, no compensation; speaker, modest), Corindus Vascular Robotics (Physician Advisory Board, stock options).
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology and the Foundation of the ASNR Symposium, June 2–7, 2018; Vancouver, British Columbia, Canada.
REFERENCES
- Received September 3, 2018.
- Accepted after revision February 4, 2019.
- © 2019 by American Journal of Neuroradiology