Abstract
BACKGROUND AND PURPOSE: Ménière disease is characterized by endolymphatic hydrops, whereas perilymphatic enhancement on MR imaging has been suggested to be of additional value in diagnosing Ménière disease. This study evaluates the presence of endolymphatic hydrops and perilymphatic enhancement in patients with Ménière disease and with other vertigo-associated inner ear pathology.
MATERIALS AND METHODS: A 3D-FLAIR sequence 4 hours after intravenous gadolinium injection was performed to visualize the endolymph and perilymph in 220 patients suspected of having Ménière disease. Patients’ ears were retrospectively categorized as having Ménière disease (probable or definite) or other vertigo-associated inner ear pathology not attributable to Ménière disease. Endolymphatic hydrops was evaluated using a visual classification system, and perilymphatic enhancement was scored both visually and quantitatively.
RESULTS: Endolymphatic hydrops was present in 137 (91.9%) of the definite Ménière disease ears and in 9 (7.0%) of the ears with other vertigo-associated inner ear pathology (P < .001). The combination of endolymphatic hydrops and visually increased perilymphatic enhancement was present in 122 (81.9%) definite Ménière disease ears compared with 4 (3.1%) ears with other vertigo-associated inner ear pathology (P < .001). This combination increases the positive predictive value from 0.94 for endolymphatic hydrops and 0.91 for perilymphatic enhancement to 0.97. The addition of measured perilymphatic enhancement leads to a moderate decrease in sensitivity from 0.92 for endolymphatic hydrops to 0.86.
CONCLUSIONS: The combination of perilymphatic enhancement and endolymphatic hydrops in patients suspected of having Ménière disease increases the positive predictive value in the diagnosis of definite Ménière disease.
ABBREVIATIONS:
- EH
- endolymphatic hydrops
- MD
- Ménière disease
- PE
- perilymphatic enhancement
- SIR
- signal intensity ratio
- VAIEP
- vertigo-associated inner ear pathology
Ménière disease (MD) is characterized by attacks of vertigo, low-frequency hearing loss, and tinnitus. In the absence of a diagnostic standard, clinical diagnostic criteria were defined by the American Academy of Otolaryngology–Head and Neck Surgery and updated into a consensus of diagnostic guidelines by the Bárány Society in 2015. This includes 2 distinct diagnostic entities: definite and probable MD, based on differences in vertigo episode duration and documented low-frequency hearing loss.1 Because key clinical symptoms overlap other clinical entities such as vestibular migraine, it remains difficult to distinguish MD from other vertigo-associated inner ear pathologies.2⇓-4
Although the etiology of MD remains unclear, endolymphatic hydrops (EH) is generally accepted as the pathologic hallmark of the disease.5 3T MR imaging after delayed intravenous gadolinium allows visualization of the endolymphatic space, with EH findings similar to histopathologic findings.6,7 However, EH is currently not part of the diagnostic criteria for definite MD. Most interesting, EH is not exclusively seen in MD but is also reported in healthy ears, monosymptomatic disease (vertigo, tinnitus, or hearing loss), and vestibular migraine.8⇓-10 Based on the potential relevance of EH in patients suspected of having MD, new diagnostic criteria have been proposed, differentiating primary hydropic ear disease (old terminology “definite MD” and “probable MD”) from hydrops due to secondary causes such as labyrinthitis or congenital hearing loss.11
Vestibular hydrops, in particular in the saccule, seems strongly correlated with MD, as demonstrated by recent MR imaging studies.12⇓-14 To improve the diagnostic accuracy in patients with suspected MD based on imaging, a few recent studies introduced perilymphatic enhancement (PE) as an additional MD-discriminating parameter.14,15 However, its presence and value in other vertigo-associated inner ear pathology (VAIEP) remains unclear.
Therefore, the purpose of this study was to evaluate the presence of EH and the additional value of PE in the diagnosis of patients with MD and in patients with other VAIEP not attributable to MD.
MATERIALS AND METHODS
Patients
From November 2017 to July 2018, two hundred twenty-seven consecutive patients who visited the Department of Otorhinolaryngology of our vertigo referral center (Haga Teaching Hospital, The Hague) with inner ear pathology and were suspected of having MD were retrospectively analyzed. Inclusion criteria were 18 years of age or older and a clinical diagnosis of definite MD or probable MD according to the 2015 updated Bárány criteria.1 Patients not fulfilling the criteria for definite or probable MD were included if they had attacks of vertigo with or without hearing loss and with or without tinnitus (other VAIEP). Exclusion criteria were prior operations of the inner ear, an insufficient medical record, or a technically inadequate MR imaging (motion artifacts, insufficient CSF suppression on 3D-FLAIR).
Patient anamnestic characteristics and hearing tests were evaluated twice by 3 otorhinolaryngologists, independently and blinded to the MR imaging results. Diagnoses were assigned for each ear separately, according to the latest Bárány criteria.1 Patients’ ears were confined to 1 of the 4 groups: definite MD, probable MD, other VAIEP, or asymptomatic (contralateral MD and other VAIEP ears). Consensus judgment was reached if evaluations were not congruent. This institutional review board–approved study was performed with a waiver of informed consent.
Imaging Protocol
All patients underwent delayed 3T MR imaging (Magnetom Skyra; Siemens, Erlangen, Germany) using a 20-channel array head coil, 4 hours after intravenous gadolinium (30 mL of gadoterate meglumine, Dotarem; Guerbet, Aulnay-sous-Bois, France). Patients were evaluated in the supine position with additional fixation between the patient’s head and the receiver coil.
High-resolution T2 sampling perfection with application-optimized contrasts by using different flip angle evolution (SPACE sequence; Siemens) images of the inner ear were obtained for anatomic reference using the following parameters: FOV = 160 mm, section thickness = 0.5 mm, TR = 1400 ms, TE = 155 ms, number of excitations = 1, flip angle = 120°, matrix = 320 × 320, bandwidth = 289 Hz/pixel, turbo factor = 96, voxel size = 0.5 × 0.5 × 0.5 mm, and acquisition time = 5 minutes. A 3D-FLAIR sequence was performed on the basis of previously reported parameters.14 In short, we used the following parameters: FOV = 190 mm, section thickness = 0.8 mm, TR = 6000 ms, TE = 177 ms, number of excitations = 1, TI = 2000 ms, flip angle = 180°, matrix = 384 × 384, bandwidth = 213 Hz/pixel, turbo factor = 28, voxel size = 0.5 × 0.5 × 0.8 mm, and acquisition time = 14 minutes.
MR Imaging Analysis
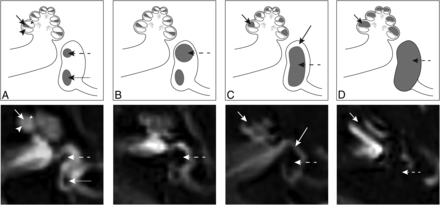
Images were analyzed using IntelliSpace PACS, Version 4.4 (Philips Healthcare, Best, the Netherlands). Images were scored for hydrops and visual signal intensity of the basal cochlear turn by 2 neuroradiologists (S.H. and O.D.V. with, respectively, 5 and 6 years of experience in MR imaging interpretation), independently and blinded to the clinical evaluation. EH was scored for the cochlea and vestibule separately using the 3 categories described by Barath;6 none, grade I (moderate), or grade II (severe). In addition to this grading system, a dilated saccule not confluent with the utricle was considered a mild isolated vestibular hydrops, according to a recently published modified scoring system.12,14 EH was considered present when either or both the cochlea and vestibule were affected (Fig 1).
Axial delayed gadolinium-enhanced 3D-FLAIR MR imaging centered at the pars inferior of the vestibule, with graphic correlations. A, Normal labyrinth: saccule (dashed arrow), utricle (dotted arrow), scala media (short arrow), scala vestibuli (small arrowhead), and scala tympani (large arrowhead). B, Mild vestibular EH: the saccule (dashed arrow) is equal in size or larger than the utricle, but not confluent. C, Moderate vestibular EH with confluence of the saccule and utricle that encompasses >50% of the vestibule (dashed arrow). A rim of surrounding perilymph remains visible (long arrow). Moderate cochlear EH with dilation of the scala media (short arrow), resulting in partial obliteration of the scala vestibuli. D, Severe vestibular EH with total effacement of the perilymphatic space in the vestibule (dashed arrow). Severe cochlear EH with complete obliteration of the scala vestibuli (short arrow).
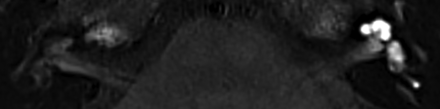
The postcontrast signal intensity of the basal cochlear turn on 3D-FLAIR images was scored both visually and quantitatively. Visually increased enhancement was defined as higher signal intensity compared with the contralateral ear or as matching the visual intensity level of patients with acute blood-labyrinth barrier breakdown/acute inflammation as shown in Fig 2. Quantitative signal intensity was scored by 1 of the authors (L.M.H.d.P.) and was calculated as the signal intensity ratio (SIR) with an oval symmetric ROI measurement in the basal cochlear turns divided by a reference measurement of 0.5 cm2 in the left middle cerebellar peduncle using the multiplanar reformation (Fig 3). Shi et al15 and Tagaya et al16 used the cerebellar hemispheres as a reference measurement, whereas Yamazaki et al17 used the medulla oblongata. In the present study, we evaluated the homogeneity of the left middle cerebellar peduncle, cerebellar hemisphere, pons, and temporal muscle (data not shown). The former proved to be the most consistent and was accordingly used as a reference to calculate the SIR.
Axial 3D-FLAIR image 4 hours after intravenous gadolinium at the midcochlear level in a patient with unilateral left-sided sudden deafness, showing diffuse perilymphatic enhancement in the cochlea and vestibule.
Axial 3D-FLAIR image 4 hours after intravenous gadolinium at the level of the basal cochlear turn of a patient with unilateral right-sided definite MD and a visually increased perilymphatic enhancement. The basal cochlear turn (oval) and the left middle cerebellar peduncle (circle) indicate the region of interest to calculate the SIR.
Statistical Analysis
Interobserver agreement between clinicians and neuroradiologists was calculated using the Cohen κ test. The Fisher exact test was used to compare the difference in the presence of EH and visual PE between the groups. For quantitative PE analysis, a generalized estimating equation was performed. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated. Among patients, a wide variability in measured PE led to the inability to quantify an absolute PE cutoff. Therefore, the added value of measured PE on EH and visual PE was calculated by adding the presence of measured PE asymmetry to the equation in patients with unilateral definite MD. The other VAIEP group is considered a control group unless specifically stated otherwise. A P value < .05 was considered significant.
RESULTS
Two hundred twenty consecutive patients from the outpatient clinic were included (127 women and 93 men; median age, 55.8 years; range, 21.5–83.2 years; median duration of sickness, 3.0 years; range, 0.005–43.0 years). Seven patients were excluded from analysis due to a prior surgical history (n = 2), an insufficient medical record (n = 3), and technically inadequate MR imaging (n = 2).
In this cohort, 19 patients were diagnosed with bilateral definite MD; 111, with unilateral definite MD (of which 5 with a contralateral other VAIEP ear); 2, with bilateral probable MD; 10, with unilateral probable MD (of which 2 with a contralateral other VAIEP ear); 43, with bilateral ears with other VAIEP; and 35, with unilateral ears with other VAIEP. Definite diagnoses of other VAIEP ears not attributable to MD are listed in Table 1.
Clinical diagnosis of ears with other VAIEP
The interobserver agreement among the clinicians to assess the diagnosis and among the neuroradiologists to assess PE and EH is shown in Table 2.
Interobserver agreement
The presence of EH in the 4 patient categories is shown in Table 3. This results in a sensitivity of 0.92 and specificity of 0.93 for definite MD ears compared with other VAIEP ears (Table 4). The MD ears (probable and definite combined) showed a sensitivity of 0.90 and specificity of 0.93, whereas the probable MD ears alone demonstrated a sensitivity and specificity of, respectively, 0.64 and 0.93. No significant differences in EH were found between the asymptomatic ears and the other VAIEP ears (respectively, 4.7% and 7.0%; Fisher exact test, P = .45).
Presence of EH and PEa
Sensitivity, specificity, PPV, and NPV in definite MD ears
The results of visually scored PE in different groups are listed in Table 3. Increased PE was more prevalent in definite and probable MD ears compared with other VAIEP ears (respectively, P < .001 and P = .003) and asymptomatic ears (both, P < .001).
In addition, a significant difference was found in the presence of PE in other VAIEP ears compared with asymptomatic ears (P < .05) as illustrated in Fig 2.
The results of the quantitative measurements of PE are shown in Table 5. The SIR of the clinically affected definite MD ears showed an increased PE of the basal cochlear turn compared with other VAIEP ears (P < .001). The probable MD ears showed no differences in the SIR compared with other VAIEP ears (P = .17) and definite MD ears (P = .06).
Generalized estimating equation for the mean SIR of PE with other VAIEP as a reference category
Four patients with unilateral definite MD showed no evidence of EH. In addition, the quantified PE was not increased in the clinically affected ear compared with the contralateral asymptomatic ear. Six of the 10 patients with unilateral definite MD with EH but without visually increased PE showed an increased measured PE in their symptomatic ear compared with their asymptomatic ear.
The combination of EH and visually increased PE was present in 81.9% of the definite MD ears, whereas it was seen in only 3.1% of the ears with other VAIEP (P < .001). Consequently, this combination increased the specificity in definite MD from 0.93 to 0.97 compared with EH alone (positive predictive value from 0.94 to 0.97 and decreased the negative predictive value from 0.91 to 0.82 and sensitivity from 0.92 to 0.82). The sensitivity increased to 0.86 when measured PE was added as shown in Table 4. The combination of EH and visually increased PE was seen in 42.9% of the probable MD ears, resulting in a sensitivity of 0.43 and specificity of 0.97.
The 4 ears with other VAIEP that demonstrated the combination of EH and PE were diagnosed with vestibular migraine (n = 3) and autoimmune inner ear disease (n = 1).
DISCUSSION
The present study shows that EH and increased PE are frequently present in patients with MD, whereas this combination is uncommon in patients with other VAIEP. Furthermore, the study emphasizes the relevance of EH as a hallmark of definite MD because vestibular or cochlear EH or both were present in 91.9% of the definite MD ears. This finding is in concordance with previously published studies with an EH prevalence ranging from 95% to 96%.6,15 In our dataset, EH is seen in only 4.7% of the asymptomatic ears, whereas percentages of 6% and 22% have been documented in contralateral MD ears in other studies.6,8 Even in patients with other VAIEP, a clinically relevant group, EH was demonstrated in only 7% of symptomatic ears. This finding is in line with previously published prevalence data in healthy control ears by Ito et al,8 in which EH (vestibular or cochlear) was present in 10% of the ears.
Recent studies suggest that vestibular hydrops is more specific for MD than cochlear hydrops.18,19 In our study, isolated EH (vestibular or cochlear) is a relatively scarce finding, though isolated vestibular hydrops is more pronounced in the definite MD group. Attyé et al12 demonstrated that half of patients with MD presented with inversion of the saccule-to-utricle ratio, whereas this was not present in the healthy subjects. This finding is in concordance with a recent published study by Shi et al.13 They showed that vestibular hydrops is more common in patients with definite MD compared with cochlear hydrops,13 though saccular hydrops may be a reflection of sensorineural hearing loss rather than MD.20,21 MR imaging evaluation of EH is robust, reflected in a high interobserver agreement ranging from 0.92 for the vestibule to 0.93 for cochlear findings (normal and abnormal). This level of agreement is higher than the clinical interobserver agreement to assess the diagnosis, which is in line with a previously published study.14
The definite MD ears showed increased PE both visually and in quantitative measurements compared with asymptomatic ears. This is in concordance with previously published studies, though references for the signal intensity ratio are different. Shi et al15 and Tagaya et al16 used the cerebellar hemispheres and found a difference in the SIR in the affected ear compared with the contralateral ear in patients with MD, whereas Yamazaki et al17 used the medulla oblongata. In our study, the left middle cerebellar peduncle was used as a reference to calculate the SIR.
The ears with other VAIEP showed an increased PE compared with asymptomatic ears. According to Kim et al,22 increased PE was also seen on the affected side with sudden sensorineural hearing loss and vestibular neuritis compared with the unaffected side. This finding shows that increased PE is a marker of disease activity in the inner ear, rather than exclusively seen in definite MD. Therefore, PE alone cannot be used to distinguish a definite MD ear from an ear with other VAIEP.
In patients with other VAIEP, the combination of EH and PE is seen in only 3.1% of the ears. This shows that this combination could play an essential role in diagnosing MD in this clinically relevant group. For example, an increased PE is seen in ears with idiopathic sensorineural hearing loss,23 whereas the detection of EH is higher in MD compared with sudden deafness24 or not even demonstrated at all.25
Our results demonstrate that PE measurements have an additional value compared with a visual score because the sensitivity increases from 0.82 to 0.86 when measured PE is added to EH, combined with visually scored PE.
The 4 patients with unilateral definite MD without EH showed no increased measured PE. However, 1 patient demonstrated an asymmetric visual PE in the vestibule with nonvisualization of the saccule, which is presumed to be the result of an intralabyrinthine fistula.26
Most interesting, 3 of the 4 ears with other VAIEP that showed both EH and PE were diagnosed with vestibular migraine, though this diagnosis as a separate clinical entity is under debate.27 However, with the current clinical criteria, it remains difficult to distinguish MD from vestibular migraine. This difficulty is in concordance with previously published studies suggesting that they share similar pathophysiological mechanisms3 or that describe a group of patients who fulfill both diagnostic criteria.28
Bernaerts et al14 demonstrated that the 2 most distinctive characteristics to distinguish MD ears from asymptomatic ears are cochlear PE and vestibular EH. Our study confirms that the combination of EH (vestibular or cochlear) and PE is distinctive for definite MD and shows, in addition, that this combination is rarely present in the ears with other VAIEP. Furthermore, PE alone cannot be used to distinguish a definite MD ear from an ear with other VAIEP.
The retrospective design of the conducted study hampers the potential to correlate the imaging findings with clinical parameters (attack frequency and time interval of last attack relative to imaging) and assess their relation with the severity of EH in the current grading systems. Furthermore, the group with other VAIEP, though clinically relevant, shows heterogeneous patient characteristics. This feature hampers the possibility to draw conclusions within this group. Another limitation is the presumption that a contralateral ear of a patient with definite MD, probable MD, and other VAIEP is considered healthy and is added to the group with asymptomatic ears.
Practical Use of MR Imaging in (Suspected) Ménière Disease
Previous studies have mainly focused on identifying inner ear abnormalities on 3D-FLAIR MR imaging by comparing symptomatic and asymptomatic ears in patients with MD.6,14,16⇓-18 These studies showed MR imaging to be highly sensitive and specific in discriminating the affected ear in patients with MD. However, considering the variable spectrum of clinical presentations in MD, a comparison with patients with other VAIEP seems relevant.9,29 The present study demonstrates the value of delayed gadolinium-enhanced 3D-FLAIR MR imaging in diagnosing MD in a cohort with a wide range of vertigo-associated inner ear diseases and shows that the combination of EH and increased PE is uncommon in patients with other VAIEP; this finding could be of particular relevance in patients in whom an atypical clinical presentation hampers a definite diagnosis, as is the case with probable MD. Although the number of patients with probable MD in our cohort is limited, 43% of these patients demonstrated the combination of EH and increased PE. On the basis of our study results, this finding suggests a (definite) MD diagnosis, which may alter treatment strategies. However, longitudinal research is necessary to evaluate clinical progression to definite MD.
In 20% of patients with MD, the vestibular and cochlear symptoms coincide after >5 years, resulting in diagnostic delay.9 Moreover, the lower interobserver agreement in the diagnoses of the clinicians compared with the hydrops scoring of neuroradiologists reflects the additional value of imaging.
Even in patients with evident, clinically definite MD, imaging is helpful in the evaluation of hydrops when conservative treatment fails, or in assessing possible bilateral hydropic disease (with unilateral symptoms) before considering, for example, sacrificing 1 ear with a destructive inner ear operation in selected cases.30,31
CONCLUSIONS
The combined presence of EH and increased PE is associated with the clinical diagnosis of definite MD and not with other VAIEP. These findings may help to differentiate patients with vertigo attributable to MD.
Acknowledgments
The authors thank Vera de Pont and Anne de Bokx for their assistance in preparing the graphics.
Footnotes
J.M. van Steekelenburg and A. van Weijnen are shared first author; H.M. Blom and S. Hammer are shared senior authors.
This work was supported by the Radiology Research Fund of the Radiological Society of the Netherlands.
Disclosures: Berit M. Verbist—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Bayer Pharmaceuticals. Sebastiaan Hammer—RELATED: Grant: Dutch Society of Radiology, Comments: A grant was awarded to assess the technical feasibility of MRI-evaluated endolymphatic hydrops and allow development of optimizing imaging techniques.
Paper previously presented at: Annual Meeting of the European Society of Neuroradiology, September 19–23, 2018, Rotterdam, the Netherlands; Annual Meeting of the European Society of Head and Neck Radiology, September 27–29, 2018, Kensington, UK; and Annual Meeting of the European Congress of Radiology, February 27–March 3, 2019, Vienna, Austria.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received September 7, 2019.
- Accepted after revision January 1, 2020.
- © 2020 by American Journal of Neuroradiology