Abstract
BACKGROUND AND PURPOSE: Conventional nonadhesive liquid embolic agents currently are the criterion standard for endovascular embolization of cerebral AVMs. However, inadequate distal penetration into the nidus and unstable proximal plug formation are the major limitations of this approach and of the currently available embolic materials. The aim of this study was to evaluate the hypothetic efficacy of combining liquid embolic agents with different properties and viscosities for use in endovascular embolization of cerebral AVMs.
MATERIALS AND METHODS: From March 2018 to March 2019, sixteen patients with cerebral AVMs (12 women, 4 men; age range, 33–61 years) underwent endovascular embolization with combined liquid embolic agents delivered serially via a single microcatheter. The procedure consists of initial embolization with PHIL 30%, followed by Menox 18 through the same microcatheter. According to the Spetzler-Martin scale, 11 (68.75%) AVMs were grades I–II, 4 (25%) were grade III, and 1 (6.25%) was grade IV. Angiographic, technical, and clinical outcomes were analyzed independently.
RESULTS: Combined PHIL and Menox embolization through the same microcatheter via 21 pedicles was performed in these 16 patients. Once the length of the reflux reached approximately 2 cm, PHIL 30% was switched to Menox 18. Antegrade flow and distal penetration of the serially applied liquid embolic agents were observed in all 16 cases. The ability to completely control the flow of the materials and avoid any dangerous proximal reflux was noted in all performed embolizations. The estimated average size reduction of the treated AVMs was 85%, ranging from 50% to 100%. Complete embolization was achieved in 10/16 or 62.5% of the cases. There was no procedure-related complication during or after the embolization. No mortality or postprocedural clinical worsening was seen. Clinical success and complete obliteration were confirmed with at least 1 follow-up angiography in 10/16 patients.
CONCLUSIONS: Serial delivery of nonadhesive liquid embolic agents via the same microcatheter was safe and effective in our study and may be a potential technique for routine AVM treatment. However, further investigations are required to validate the safety and the efficacy of the method.
ABBREVIATIONS:
- DMSO
- dimethyl-sulfoxide
- EVOH
- ethylene-vinyl alcohol copolymer
- LEA
- liquid embolic agent
Liquid embolic agents are primarily used for treatment of cerebral AVMs.1 The most widely used liquid embolic agents are the nonadhesive ethylene-vinyl alcohol copolymers (EVOHs): Onyx (Covidien, Irvine, California), SQUID (Emboflu, Gland, Switzerland), Menox (Meril Life Sciences, Gujarat India), and polyhydroxyethylmethacrylate copolymer—Precipitating Hydrophobic Injectable Liquid (PHIL; MicroVention, Tustin, California), along with the well-known n-BCA.2-4 The major limitation of the conventional EVOH embolization technique is that on some occasions, it is difficult to control the reflux of the material at the level of microcatheter tip and the formation of a stable proximal plug. Establishing a stable proximal plug is crucial because it allows better distal antegrade nidal penetration. Technically speaking, EVOHs with extra-low viscosity could improve the distal penetration of the embolic agent. Currently, the only commercially available extra-low-viscosity formulation of EVOH is SQUID 12.5
Initial experience with the recently introduced liquid embolic agent PHIL has shown that this agent may, due to its intrinsic properties, address the limitation of its nonadhesive EVOH competitors in terms of proximal plug formation.6
We sought to explore whether 2 different, serially applied nonadhesive liquid embolic agents via the same microcatheter could increase the embolization success rate of cerebral AVMs. We hypothesized that using liquid embolic agents with different properties and viscosities could improve the AVM embolization with less proximal reflux. The technical aspects of this approach were evaluated as were immediate clinical and angiographic results.
MATERIALS AND METHODS
The study protocol was reviewed and approved according to the local institutional policy, and appropriate consent was obtained from each patient. From March 2018 to March 2019, in a single-center institution, a total of 16 patients with cerebral AVMs underwent endovascular embolization with combined liquid embolic agents (LEAs) serially delivered via a single microcatheter. All patient demographics, AVM angioarchitecture, procedural details, pre- and postprocedural mRS scores, and clinical data were collected (Table 1). The study population consisted of 12 women and 4 men, with ages ranging from 33 to 61 years. The most common clinical presentation was hemorrhage in 75% of patients (12/16), with the remaining 25% (4/16) presenting with headaches and epileptic seizures. Based on the size, eloquence, and venous drainage of the lesions per the Spetzler-Martin scale, 11 (68.75%) AVMs were grades I–II, 4 (25%) were grade III, and 1 (6.25%) was grade IV. Low-grade AVMs (Spetzler-Martin grades I and II) were intended to be definitively treated, while staged endovascular embolization was planned for larger AVMs, rendering them suitable for subsequent microsurgical resection. None of the included patients had any previous microsurgical or endovascular treatment.
Baseline characteristics of the treated patients
Description of the Technique
All procedures were performed with the patient under general anesthesia in a dedicated neuroangiography suite. During the procedures, systolic blood pressure was maintained between 80 and 100 mm Hg to prevent any possible migration of embolic material to the venous compartment of the AVM. Catheterization of the right distal radial artery was performed in all 16 patients. A 6F guiding catheter was then introduced into the appropriate feeding artery over an exchange-length wire using standard techniques. The choice of arterial feeders most suitable for intermixed embolization was made by the treating physician on a case-by-case basis. Dimethyl-sulfoxide (DMSO)-compatible Apollo microcatheters (Covidien) were delivered over a 0.010- or 0.008-inch microwire into the selected feeding pedicle and its desired segment with the tip positioned as close as possible to the nidus of the AVM. The hub lumen and the dead space of the microcatheter were then primed with 0.25 mL of DMSO.
The basis of the embolization technique was to first perform primary injections with PHIL 30% to optimize the initial plug formation. As soon as the length of injected PHIL reached 1.5 cm or was close to the proximal marker of the microcatheter, Menox 18 was applied as a second embolic agent via the same microcatheter. Additional DMSO was not injected between applications of the 2 liquid embolic agents. Because a solid proximal plug was already formed, Menox, due to its different intrinsic properties, was able to penetrate more distally and thus achieve an optimized AVM embolization via the same microcatheter. On completion of the endovascular embolization, control contrast injections were performed through the guide catheter and the microcatheter was subsequently removed from the pedicle under fluoroscopy. This technique was performed in all cases (Fig 1). No pressure-cooker technique or any other antireflux modifications were used in this cohort.7
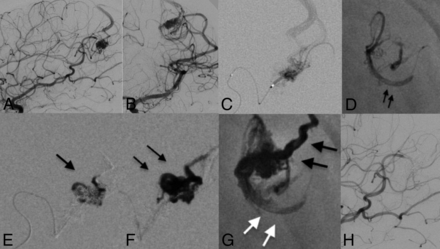
A 35-year-old female patient who presented with left-sided headache and seizures. DSA revealed a left parietal (A and B) AVM supplied by the left MCA. The AVM was drained by the superficial cortical vein to the superior sagittal sinus. An Apollo microcatheter was positioned into a distal arterial feeder arising from the left MCA (C). A PHIL 30 plug was created with 2 rounds of injections (D, arrows). After 1.2 mL of PHIL 30 was injected into the AVM, embolization was continued with Menox 18 (E and F) to ensure successful distal and continuous penetration. No further proximal reflux was observed (G). Note the different radiopacities of the used liquid embolic agents (black arrows, Menox 18; white arrows, PHIL 30.) At the end of the procedure, 100% size reduction was achieved by delivering a total amount of 2.7 mL of embolic material from 1 feeder during 1 session (H).
At the end of each embolization session and for each microcatheter used, the amount of traction force required to successfully retrieve the catheter was individually assessed. The perceived difficulty (eg, easy, moderate, strong) was subjectively documented by the main operator following every microcatheter removal.
Distal detachable tips of the microcatheters used were carefully examined under stereomicroscopy for any evidence of adherent or mixed embolic material fragments.
Angiographic results were determined by estimating the percentage of nidus reduction observed in the last intraprocedural angiogram. All angiograms were blindly and independently reviewed by 2 outside interventionists. Final verdict and complete interpretation of the data were reached through a consensus session.
Study Outcome Measures
As per our institutional routine protocol, the first follow-up with DSA combined with clinical evaluation was performed 6 months after the treatment. A cranial MR imaging and clinical assessment were performed on discharge after each endovascular session and on each follow-up if considered mandatory. The neurologic examination was performed by a neurologist or a certified stroke nurse and recorded using the mRS.
Primary End Points
The primary end points were complete nidal occlusion of Spetzler-Martin grade I–II AVMs, with the rate of favorable clinical outcomes defined as mRS 0–2 at 30 days after embolization.
Secondary End Points
Secondary outcomes included either intraprocedural or postprocedural intervention-related complications leading to permanent neurologic deficits. Other secondary outcomes included parent vessel injury, extravasation of the applied LEAs, rerupture of the AVM, the degree of the nidal obliteration, and the occurrence of intervention-related stroke or death. Technical complications were defined as unsuccessful microcatheter retrieval, microcatheter occlusion, and any adverse physiologic changes due to the combination of both agents.
RESULTS
Combined PHIL and Menox embolization was performed in all 16 patients. Fast and solid proximal plug formation was achieved in all cases via initial applications of PHIL 30%. Proximal plug formation with PHIL 30% was performed without difficulty in 21 pedicles, and the embolization proceeded accordingly. Successful and continuous application of the second embolic agent, Menox 18, was performed in all patients. Antegrade flow of the EVOH material into the nidal components was observed in all 16 cases. The mean volume of PHIL used was 0.9 mL (range, 0.7–2.3 mL), and the mean volume of EVOH injected was 4.1 mL (range, 1.2–6 mL). We noted mean injections pause times of 0.4 seconds for the PHIL and 1.1 seconds for the EVOH with a mean time under fluoroscopy of just 41 minutes per procedure. Technical results are summarized in Table 2.
Embolization characteristics and technical resultsa
No microcatheter remained irreversibly trapped in the plug, and no technical complications such as microcatheter occlusion or rapid tantalum sedimentation occurred. Six detachable distal tips of the used microcatheters were successfully retrieved. Among those 6 microcatheters, in 2 of them, adherent microscopic particles of the liquid embolic cast and structural crack scratches were noted over the most distal portion of the tip.
No adverse physiologic changes such as vessel injury, embolic agent extravasation, or microcatheter rupture were observed.
Angiographic Outcomes and Follow-Up Examinations
AVM volume was calculated using the method of Pasqualin et al.8 Adequate nidal penetration was achieved in all cases. The estimated average size reduction of the treated AVMs was 85%, ranging from 50% to 100%. We achieved complete angiographic obliteration of the nidus in 10/16 (62.5%) patients after the initial session with serially combined LEAs, all of whom had small AVMs (Spetzler-Martin grade I or II) (Fig 2). Favorable clinical outcome and complete obliteration were confirmed with at least 1 follow-up angiography after a mean of 5.1 months (range, 3–6 months) in each of the 10 patients.
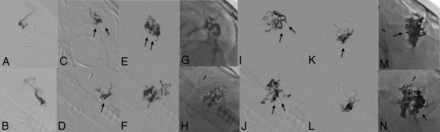
Step-by-step embolization of a ruptured left-sided parietal AVM, Spetzler-Martin grade II. The tip of the microcatheter was placed into the nidus (A and B). Initial injections of PHIL 30 (C, D, E, F, G, and H; arrows) were needed to create a stable proximal plug. The embolic material was switched with Menox 18 to successfully achieve distal and continuous nidal penetration (I–L; arrows). Note the distal extent of the Menox agent (M, N, arrow) and the different radio-opacities of the applied LEAs.
Partial embolization was achieved in 6/16 (37.5%) patients. One of those patients underwent microsurgical resection of the nidus due to enlargement of the intraparenchymal hematoma that was present during radiologic examination at admission.
Planned embolization via the same method was performed for the remaining 5/16 (31.25%) patients to achieve sufficient AVM nidal occlusion suitable for subsequent radiosurgery or surgical resection.
There was no procedure-related complication during or after the embolization. No mortality or worsening mRS postprocedurally was seen in this cohort.
DISCUSSION
Today, dimethyl-sulfoxide–based nonadhesive embolic agents are the main tool used for the multimodality treatment of cerebral AVMs.9 EVOH was pioneered in the neurointerventional field and quickly became proclaimed the criterion standard for curative AVM embolization.10 This liquid embolic material is thought to be an easily manageable agent. Its greatest advantage of slowly controllable embolization and the ability to initiate and interrupt injections may reduce the risk of any dangerous migration of the embolic mass. However, the major limitation of EVOH is that its nonadhesive nature limits control over the direction of polymer flow and can lead to excessive reflux, which may result in incomplete nidal embolization or dangerous proximal backflow and obstruction of normal arterial feeders. Usually, this issue can be addressed by intentionally allowing the agent to cover a small portion of the detachable tip of the microcatheter, creating a proximal “plug.”11 This process often requires multiple short cycles of repeat injections, thereby increasing the total duration of the procedure as well as the risk of vessel rupture or rapid tantalum sedimentation inside the microcatheter.12 The waiting time between applications of the agent to ensure an appropriate degree of hardening of the LEA is important because not waiting long enough can lead to premature embolization of the feeding artery. On the other hand, the flow pattern through the nidus can be somehow redirected by allowing the already-injected material to harden and thus creating a new low-resistance channel.13 All of these shortcomings are associated with the effect of the plug and properties of the EVOH technique.
The polylactide-co-glycolide and polyhydroxyethylmethacrylate copolymer PHIL is a nonadhesive and inherently radio-opaque LEA. Initial experience with this agent has revealed the potential advantages of this LEA over the standard EVOH technique. Reported differences include the ability to create a faster and stable proximal plug, a constant degree of distal nidal penetration, and consistent visibility due to the presence of iodine as its radio-opacity component. The material is available in 3 different concentrations and viscosities: PHIL 25, 30, and 35. Higher concentrations are preferred in the presence of a fistulous component, whereas lower concentrations are used to achieve better distal penetration. It has already been demonstrated that the successful outcome of embolization can be based on the effect of the proximal plug and the viscosity of the material used.14,15
Both Menox 18 and PHIL are DMSO-based liquid embolic agents that require DMSO-compatible microcatheters to enable a safe and efficient embolization process. Special emphasis is placed on the need for deliberation when choosing the most appropriate embolic material. Depending on the specific angioarchitecture and the geometry of the feeding arteries, use or even the combination of liquid embolic agents is often required. In our experience, combined injections of PHIL and Menox via the same microcatheter have been technically possible and safe, offering the advantage of switching between agents without the additional application of DMSO. The lesser layering effect and higher viscosity of PHIL 30 offer better properties of the proximal plug, thus ensuring successful distal penetration of Menox 18, which has 50% lower viscosity (36 cSt, PHIL 30, versus 18 cSt, Menox 18). More specifically, the initial injection of PHIL 30 creates a proximal stop that successfully blocks the flow and creates a distinctive change in the pressure gradient within the nidus, thus supporting the forward, more distal penetration of Menox due to its lower viscosity. We believe this may limit potentially dangerous proximal backflow and decrease the amount of embolic agent used. We noted a mean time under fluoroscopy of only 41 minutes and relatively shorter EVOH pause times.
From our routinely collected data, we performed direct comparisons among the Menox or PHIL infiltration times using this approach versus the conventional method. The reported injection pause times and the mean fluoroscopy times were relatively shorter than those we observe on a daily basis in comparison with injections of EVOH or PHIL as stand-alone liquid embolic agents.16,17 However, direct head-to-head comparison is difficult to perform with great accuracy because each AVM has distinct anatomic and flow characteristics.
Serial application of EVOH and PHIL via the same microcatheter was reported by Koçer et al6 in a single case scenario during their preliminary experience with PHIL. The authors did not report any technical complications or adverse physiologic changes due to the combination of both agents.
Recently, Xu et al18 described an efficient method of combining regular and diluted viscosities of EVOH to treat cerebral AVMs. The proposed method consists of initial embolization with regular-viscosity formulations of EVOH, followed by injection of a lower viscosity mixture of 1.5 mL of EVOH diluted with 0.5 mL of DMSO through the same microcatheter. The technique was performed in 15 patients with a reported average of 90% (range, 55%–100%) estimated size reduction. In 6 patients, the AVMs were completely obliterated. Control over the proximal reflux and success of the technique was achieved in 80% of the cases.
In regards to fluid resistance to deformation, extra-low-viscosity formulations of the established nonadhesive LEAs, PHIL 25% low viscosity and SQUID 12. As mentioned above, of the commercially available precipitating, nonadhesive embolic agents, SQUID 12 is the only extra-low-viscosity version so far with a growing body of literature supporting the effectiveness of this agent when used for embolization of vascular malformations.19,20 Several experimental studies investigated the improved features of PHIL 25% low-viscosity and SQUID 12 formulations.21,22 Both reports confirmed the embolization extent and the distal penetration to be higher for the low-viscosity agents compared with the standard ones. All investigated embolic agents demonstrated optimal flow control and a significantly lower amount of reflux. This aspect can be highly relevant in clinical practice. However, the authors suggested conceivable drawbacks of extra-low-viscosity LEAs in terms of early distal embolization, thus increasing the risk of premature obliteration of the draining veins or unintentional embolization of vital vasculature. Nevertheless, the yielded technical results suggest that for selected types of vascular lesions, the low-viscosity LEAs can be the preferred agents over the currently available standard-viscosity LEAs (eg, Onyx 18, SQUID 18, Menox 18, and PHIL 25%).
Several antireflux modifications are being developed to gain better control over the flow during embolization. The so-called pressure-cooker technique is designed to create an antireflux plug by trapping the detachable part of the microcatheter used for Onyx delivery via coils and glue. Successful execution of this method is reported to obtain a better forceful and controlled EVOH embolization.
The emergence of DMSO-compatible balloons provides better reflux control and improved flow conditions when using nonadhesive LEAs, allowing the agents to travel more distally.23 Difficulties in navigation in distal arterial sites and the risks of vessel rupture due to balloon overinflation exist in this technique.24 Using double-lumen balloon catheters with even smaller diameters may reduce the technical complications and increase the embolization rates.25 In this context, commercially available prototypes of extra-small balloon microcatheters can be extremely advantageous and make untreatable lesions curable.
Our initial experience with combining nonadhesive liquid embolic agents with different viscosities for AVM treatment yielded promising results. The average estimated size reduction of the nidus was 85%. Total endovascular obliteration in 1 endovascular session was achieved in more than half of the included patients (10/16). In our initial-but-limited experience with this method, we did not observe an increased risk of inadequate nidal embolization, retraction failures, or any occasions in which the used LEAs precipitated and blocked the microcatheter.
Limitations
Despite being the largest clinical study of the treatment of cerebral AVMs with combined liquid embolic agents delivered via the same microcatheter reported to date, our study has several important limitations. First, this is a single-center experience, and the technical results are limited by the authors’ individual techniques and experiences. Second, the sample is relatively small. Thus, the results of our study should be interpreted with caution because they may not be widely applicable to general practice. Last, pharmacoeconomics in developing countries holds promise for patients with certain neurovascular diseases, particularly those who require multiple treatment modalities. Unfortunately, this issue was the only reason for particularly choosing Menox 18 over the other available EVOHs. The same is true for the use of PHIL 30% for the initial plug formation followed by further embolization with PHIL 25%.
CONCLUSIONS
Combined nonadhesive liquid embolic agents delivered via the same microcatheter have the potential to facilitate and increase the success rate of endovascular embolization of cerebral AVMs while carrying risks similar to those of other available approaches. Due to the relatively small size of our study, further investigations are required to validate the safety and the efficacy of the method.
References
- Received November 5, 2019.
- Accepted after revision January 10, 2020.
- © 2020 by American Journal of Neuroradiology